Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake
- PMID: 21489572
- PMCID: PMC3137694
- DOI: 10.1016/j.metabol.2011.01.008
Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake
Abstract
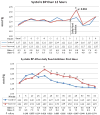
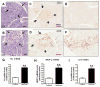
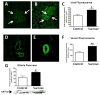
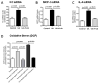
Fructose induces metabolic syndrome in rats; but studies have been criticized for using high concentrations of fructose that are not physiologic, for using only pure fructose, and for not controlling for energy intake. We tested the hypothesis that a 40% sucrose diet (containing 20% fructose) might induce features of metabolic syndrome in male breeder rats independent of excess energy intake. Male Sprague-Dawley breeder rats were pair fed 40% sucrose or isocaloric starch diet for 4 months and evaluated for metabolic syndrome and diabetes. In vitro studies were performed in rat insulinoma cells (RIN-m5F) exposed to uric acid, and markers of inflammation were assessed. Rats fed a 40% sucrose diet developed accelerated features of metabolic syndrome with up-regulation of fructose-dependent transporter Glut5 and fructokinase. Fatty liver and low-grade pancreatic inflammation also occurred. Uric acid was found to stimulate inflammatory mediators and oxidative stress in islet cells in vitro. Sucrose, at concentrations ingested by a subset of Americans, can accelerate metabolic syndrome, fatty liver, and type 2 diabetes mellitus in male breeder rats; and the effects are independent of excess energy intake.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr R Johnson, Dr Nakagawa, and Dr Lanaspa have patent applications related to lowering uric acid or blocking fructose metabolism in the treatment of metabolic syndrome. Dr Johnson also has a book, the Sugar Fix (Rodale, 2008; and Simon and Schuster, 2009) that discusses the potential role of fructose in the obesity epidemic.
Figures



References
-
- Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutrition reviews. 2005;63:133–157. - PubMed
-
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. - PubMed
-
- White JS. No unique role for fructose sweeteners in obesity or cardiorenal disease. The American journal of clinical nutrition. 2008;87:1062–1063. author reply 1063–1066. - PubMed
-
- Jones JM. Dietary sweeteners containing fructose: overview of a workshop on the state of the science. The Journal of nutrition. 2009;139:1210S–1213S. - PubMed
-
- Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

