Successive microsporogenesis affects pollen aperture pattern in the tam mutant of Arabidopsis thaliana
- PMID: 21489970
- PMCID: PMC3101141
- DOI: 10.1093/aob/mcr074
Successive microsporogenesis affects pollen aperture pattern in the tam mutant of Arabidopsis thaliana
Abstract
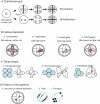
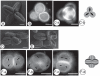
Background and aims: The tam (tardy asynchronous meiosis) mutant of Arabidopsis thaliana, which exhibits a modified cytokinesis with a switch from simultaneous to successive cytokinesis, was used to perform a direct test of the implication of cytokinesis in aperture-pattern ontogeny of angiosperm pollen grains. The aperture pattern corresponds to the number and arrangement of apertures (areas of the pollen wall permitting pollen tube germination) on the surface of the pollen grain.
Methods: A comparative analysis of meiosis and aperture distribution was performed in two mutant strains of arabidopsis: quartet and quartet-tam.
Key results: While the number of apertures is not affected in the quartet-tam mutant, the arrangement of the three apertures is modified compared with the quartet, resulting in a different aperture pattern.
Conclusions: These results directly demonstrate the relationship between the type of sporocytic cytokinesis and pollen aperture-pattern ontogeny.
Figures

References
-
- Arens K. Prova de calose por meio da microscopia a luz fluorescente e aplicações do metodo. Lilloa. 1949;18:71–75.
-
- Banks H, Stafford P, Crane P. Aperture variation in the pollen of Nelumbo (Nelumbonaceae) Grana. 2007;46:157–163.
-
- Blackmore S, Barnes SH. Garside's rule and the microspore tetrads of Grevillea rosmarinifolia A. Cunningham and Dryandra polycephala Bentham (Proteaceae) Review of Palaeobotany and Palynology. 1995;85:111–121.
-
- Blackmore S, Crane P. The systematic implications of pollen and spore ontogeny. In: Humpries CJ, editor. Ontogeny and systematics. New York, NY: Columbia University Press; 1988. pp. 83–115.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

