Serine/threonine kinase 17A is a novel p53 target gene and modulator of cisplatin toxicity and reactive oxygen species in testicular cancer cells
- PMID: 21489989
- PMCID: PMC3103316
- DOI: 10.1074/jbc.M111.218040
Serine/threonine kinase 17A is a novel p53 target gene and modulator of cisplatin toxicity and reactive oxygen species in testicular cancer cells
Abstract
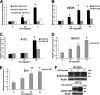
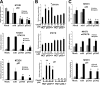
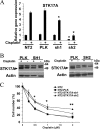
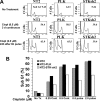
Testicular cancer is highly curable with cisplatin-based therapy, and testicular cancer-derived human embryonal carcinoma (EC) cells undergo a p53-dominant transcriptional response to cisplatin. In this study, we have discovered that a poorly characterized member of the death-associated protein family of serine/threonine kinases, STK17A (also called DRAK1), is a novel p53 target gene. Cisplatin-mediated induction of STK17A in the EC cell line NT2/D1 was prevented with p53 siRNA. Furthermore, STK17A was induced with cisplatin in HCT116 and MCF10A cells but to a much lesser extent in isogenic p53-suppressed cells. A functional p53 response element that binds endogenous p53 in a cisplatin-dependent manner was identified 5 kb upstream of the first coding exon of STK17A. STK17A is not present in the mouse genome, but the closely related gene STK17B is induced with cisplatin in mouse NIH3T3 cells, although this induction is p53-independent. Interestingly, in human cells containing both STK17A and STK17B, only STK17A is induced with cisplatin. Knockdown of STK17A conferred resistance to cisplatin-induced growth suppression and apoptotic cell death in EC cells. This was associated with the up-regulation of detoxifying and antioxidant genes, including metallothioneins MT1H, MT1M, and MT1X that have previously been implicated in cisplatin resistance. In addition, knockdown of STK17A resulted in decreased cellular reactive oxygen species, whereas STK17A overexpression increased reactive oxygen species. In summary, we have identified STK17A as a novel direct target of p53 and a modulator of cisplatin toxicity and reactive oxygen species in testicular cancer cells.
Figures


References
-
- Houldsworth J., Korkola J. E., Bosl G. J., Chaganti R. S. (2006) J. Clin. Oncol. 24, 5512–5518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

