Recognition of multivalent histone states associated with heterochromatin by UHRF1 protein
- PMID: 21489993
- PMCID: PMC3129210
- DOI: 10.1074/jbc.M111.234104
Recognition of multivalent histone states associated with heterochromatin by UHRF1 protein
Abstract
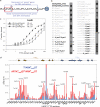
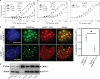
Histone modifications and DNA methylation represent two layers of heritable epigenetic information that regulate eukaryotic chromatin structure and gene activity. UHRF1 is a unique factor that bridges these two layers; it is required for maintenance DNA methylation at hemimethylated CpG sites, which are specifically recognized through its SRA domain and also interacts with histone H3 trimethylated on lysine 9 (H3K9me3) in an unspecified manner. Here we show that UHRF1 contains a tandem Tudor domain (TTD) that recognizes H3 tail peptides with the heterochromatin-associated modification state of trimethylated lysine 9 and unmodified lysine 4 (H3K4me0/K9me3). Solution NMR and crystallographic data reveal the TTD simultaneously recognizes H3K9me3 through a conserved aromatic cage in the first Tudor subdomain and unmodified H3K4 within a groove between the tandem subdomains. The subdomains undergo a conformational adjustment upon peptide binding, distinct from previously reported mechanisms for dual histone mark recognition. Mutant UHRF1 protein deficient for H3K4me0/K9me3 binding shows altered localization to heterochromatic chromocenters and fails to reduce expression of a target gene, p16(INK4A), when overexpressed. Our results demonstrate a novel recognition mechanism for the combinatorial readout of histone modification states associated with gene silencing and add to the growing evidence for coordination of, and cross-talk between, the modification states of H3K4 and H3K9 in regulation of gene expression.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

