Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi
- PMID: 21490058
- PMCID: PMC3082299
- DOI: 10.1242/dev.060020
Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi
Abstract
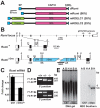
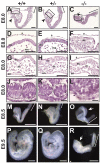
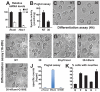
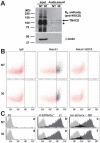
Protein O-glucosylation is a conserved post-translational modification that occurs on epidermal growth factor-like (EGF) repeats harboring the C(1)-X-S-X-P-C(2) consensus sequence. The Drosophila protein O-glucosyltransferase (Poglut) Rumi regulates Notch signaling, but the contribution of protein O-glucosylation to mammalian Notch signaling and embryonic development is not known. Here, we show that mouse Rumi encodes a Poglut, and that Rumi(-/-) mouse embryos die before embryonic day 9.5 with posterior axis truncation and severe defects in neural tube development, somitogenesis, cardiogenesis and vascular remodeling. Rumi knockdown in mouse cell lines results in cellular and molecular phenotypes of loss of Notch signaling without affecting Notch ligand binding. Biochemical, cell culture and cross-species transgenic experiments indicate that a decrease in Rumi levels results in reduced O-glucosylation of Notch EGF repeats, and that the enzymatic activity of Rumi is key to its regulatory role in the Notch pathway. Genetic interaction studies show that removing one copy of Rumi in a Jag1(+/-) (jagged 1) background results in severe bile duct morphogenesis defects. Altogether, our data indicate that addition of O-glucose to EGF repeats is essential for mouse embryonic development and Notch signaling, and that Jag1-induced signaling is sensitive to the gene dosage of the protein O-glucosyltransferase Rumi. Given that Rumi(-/-) embryos show more severe phenotypes compared to those displayed by other global regulators of canonical Notch signaling, Rumi is likely to have additional important targets during mammalian development.
Figures




References
-
- Bakker H., Oka T., Ashikov A., Yadav A., Berger M., Rana N. A., Bai X., Jigami Y., Haltiwanger R. S., Esko J. D., et al. (2009). Functional UDP-xylose transport across the endoplasmic reticulum/Golgi membrane in a Chinese hamster ovary cell mutant defective in UDP-xylose Synthase. J. Biol. Chem. 284, 2576-2583 - PMC - PubMed
-
- Bulman M. P., Kusumi K., Frayling T. M., McKeown C., Garrett C., Lander E. S., Krumlauf R., Hattersley A. T., Ellard S., Turnpenny P. D. (2000). Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat. Genet. 24, 438-441 - PubMed
-
- Busch C., Hofmann F., Gerhard R., Aktories K. (2000). Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. J. Biol. Chem. 275, 13228-13234 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

