An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses
- PMID: 21490090
- PMCID: PMC3126508
- DOI: 10.1128/JVI.00073-11
An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses
Abstract
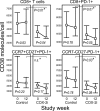
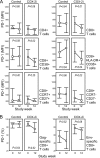
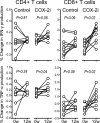
Chronic HIV infection is characterized by chronic immune activation and dysfunctional T cells with elevated intracellular cyclic AMP (cAMP), which inhibits the T cell activation capability. cAMP may be induced by prostaglandin E(2) following lipopolysaccharide (LPS)-induced upregulation of cyclooxygenase type 2 (COX-2) in monocytes due to the elevated LPS levels in patients with chronic HIV infection. This hypothesis was tested using celecoxib, a COX-2 inhibitor, for 12 weeks in HIV-infected patients without antiretroviral treatment in a prospective, open, randomized exploratory trial. Thirty-one patients were randomized in the trial; 27 completed the study, including 13 patients on celecoxib. Celecoxib reduced chronic immune activation in terms of CD38 density on CD8(+) T cells (-24%; P = 0.04), IgA levels (P = 0.04), and a combined score for inflammatory markers (P < 0.05). Celecoxib further reduced the inhibitory surface receptor programmed death 1 (PD-1) on CD8(+) T cells (P = 0.01), including PD-1 on the HIV Gag-specific subset (P = 0.02), enhanced the number of CD3(+) CD4(+) CD25(+) CD127(lo/-) Treg or activated cells (P = 0.02), and improved humoral memory recall responses to a T cell-dependent vaccine (P = 0.04). HIV RNA (P = 0.06) and D dimers (P = 0.07) tended to increase in the controls, whereas interleukin-6 (IL-6) possibly decreased in the treatment arm (P = 0.10). In conclusion, celecoxib downmodulated the immune activation related to clinical progression of chronic HIV infection and improved T cell-dependent functions in vivo.
Figures



References
-
- Aandahl E. M., et al. 1998. Protein kinase A type I antagonist restores immune responses of T cells from HIV-infected patients. FASEB J. 12:855–862 - PubMed
-
- Aandahl E. M., et al. 1999. Additive effects of IL-2 and protein kinase A type I antagonist on function of T cells from HIV-infected patients on HAART. AIDS 13:F109–F114 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

