Immunization with recombinant HLA classes I and II, HIV-1 gp140, and SIV p27 elicits protection against heterologous SHIV infection in rhesus macaques
- PMID: 21490092
- PMCID: PMC3126489
- DOI: 10.1128/JVI.00129-11
Immunization with recombinant HLA classes I and II, HIV-1 gp140, and SIV p27 elicits protection against heterologous SHIV infection in rhesus macaques
Abstract
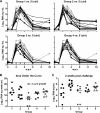
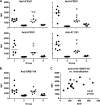
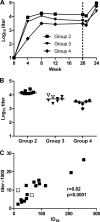
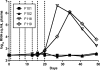
Major histocompatibility complex (MHC) molecules expressed on the surface of human immunodeficiency virus (HIV) are potential targets for neutralizing antibodies. Since MHC molecules are polymorphic, nonself MHC can also be immunogenic. We have used combinations of novel recombinant HLA class I and II and HIV/simian immunodeficiency virus (SIV) antigens, all linked to dextran, to investigate whether they can elicit protective immunity against heterologous simian/human immunodeficiency virus (SHIV) challenge in rhesus macaques. Three groups of animals were immunized with HLA (group 1, n = 8), trimeric YU2 HIV type 1 (HIV-1) gp140 and SIV p27 (HIV/SIV antigens; group 2, n = 8), or HLA plus HIV/SIV antigens (group 3, n = 8), all with Hsp70 and TiterMax Gold adjuvant. Another group (group 4, n = 6) received the same vaccine as group 3 without TiterMax Gold. Two of eight macaques in group 3 were completely protected against intravenous challenge with 18 50% animal infective doses (AID(50)) of SHIV-SF162P4/C grown in human cells expressing HLA class I and II lineages represented in the vaccine, while the remaining six macaques showed decreased viral loads compared to those in unimmunized animals. Complement-dependent neutralizing activity in serum and high levels of anti-HLA antibodies were elicited in groups 1 and 3, and both were inversely correlated with the plasma viral load at 2 weeks postchallenge. Antibody-mediated protection was strongly supported by the fact that transfer of pooled serum from the two challenged but uninfected animals protected two naïve animals against repeated low-dose challenge with the same SHIV stock. This study demonstrates that immunization with recombinant HLA in combination with HIV-1 antigens might be developed into an alternative strategy for a future AIDS vaccine.
Figures


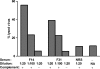
References
-
- Brown E. J. 1991. Complement receptors and phagocytosis. Curr. Opin. Immunol. 3:76–82 - PubMed
-
- Carroll M. C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986 - PubMed
-
- Chan W. L., et al. 1995. Immunization with class I human histocompatibility leukocyte antigen can protect macaques against challenge infection with SIVmac-32H. AIDS 9:223–228 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

