Gambogic acid inhibits STAT3 phosphorylation through activation of protein tyrosine phosphatase SHP-1: potential role in proliferation and apoptosis
- PMID: 21490133
- PMCID: PMC3131433
- DOI: 10.1158/1940-6207.CAPR-10-0340
Gambogic acid inhibits STAT3 phosphorylation through activation of protein tyrosine phosphatase SHP-1: potential role in proliferation and apoptosis
Retraction in
-
Retraction: Gambogic Acid Inhibits STAT3 Phosphorylation through Activation of Protein Tyrosine Phosphatase SHP-1: Potential Role in Proliferation and Apoptosis.Cancer Prev Res (Phila). 2018 Sep;11(9):593. doi: 10.1158/1940-6207.CAPR-18-0235. Cancer Prev Res (Phila). 2018. PMID: 30181122 Free PMC article. No abstract available.
Abstract
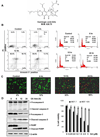
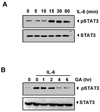
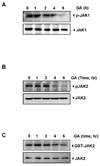
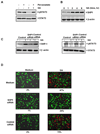
The transcription factor, STAT3, is associated with proliferation, survival, and metastasis of cancer cells. We investigated whether gambogic acid (GA), a xanthone derived from the resin of traditional Chinese medicine, Garcinia hanburyi (mangosteen), can regulate the STAT3 pathway, leading to suppression of growth and sensitization of cancer cells. We found that GA induced apoptosis in human multiple myeloma cells that correlated with the inhibition of both constitutive and inducible STAT3 activation. STAT3 phosphorylation at both tyrosine residue 705 and serine residue 727 was inhibited by GA. STAT3 suppression was mediated through the inhibition of activation of the protein tyrosine kinases Janus-activated kinase 1 (JAK1) and JAK2. Treatment with the protein tyrosine phosphatase (PTP) inhibitor pervanadate reversed the GA-induced downregulation of STAT3, suggesting the involvement of a PTP. We also found that GA induced the expression of the PTP SHP-1. Deletion of the SHP-1 gene by siRNA suppressed the ability of GA to inhibit STAT3 activation and to induce apoptosis, suggesting the critical role of SHP-1 in its action. Moreover, GA downregulated the expression of STAT3-regulated antiapoptotic (Bcl-2, Bcl-xL, and Mcl-1), proliferative (cyclin D1), and angiogenic (VEGF) proteins, and this correlated with suppression of proliferation and induction of apoptosis. Overall, these results suggest that GA blocks STAT3 activation, leading to suppression of tumor cell proliferation and induction of apoptosis.
Figures

Similar articles
-
Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells.Int J Cancer. 2010 Jul 15;127(2):282-92. doi: 10.1002/ijc.25059. Int J Cancer. 2010. PMID: 19937797 Free PMC article.
-
Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases.Cancer Lett. 2014 Apr 1;345(1):140-8. doi: 10.1016/j.canlet.2013.12.008. Epub 2013 Dec 11. Cancer Lett. 2014. PMID: 24333736
-
γ-Tocotrienol but not γ-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents.J Biol Chem. 2010 Oct 22;285(43):33520-33529. doi: 10.1074/jbc.M110.158378. Epub 2010 Aug 18. J Biol Chem. 2010. Retraction in: J Biol Chem. 2016 Aug 5;291(32):16922. doi: 10.1074/jbc.A110.158378. PMID: 20720018 Free PMC article. Retracted.
-
β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase.Mol Carcinog. 2014 Oct;53(10):793-806. doi: 10.1002/mc.22035. Epub 2013 Jun 13. Mol Carcinog. 2014. PMID: 23765383
-
Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells.Phytomedicine. 2016 May 15;23(5):566-77. doi: 10.1016/j.phymed.2016.02.011. Epub 2016 Feb 27. Phytomedicine. 2016. PMID: 27064016
Cited by
-
Retraction: Gambogic Acid Inhibits STAT3 Phosphorylation through Activation of Protein Tyrosine Phosphatase SHP-1: Potential Role in Proliferation and Apoptosis.Cancer Prev Res (Phila). 2018 Sep;11(9):593. doi: 10.1158/1940-6207.CAPR-18-0235. Cancer Prev Res (Phila). 2018. PMID: 30181122 Free PMC article. No abstract available.
-
SHP1-mediated cell cycle redistribution inhibits radiosensitivity of non-small cell lung cancer.Radiat Oncol. 2013 Jul 10;8:178. doi: 10.1186/1748-717X-8-178. Radiat Oncol. 2013. PMID: 23842094 Free PMC article.
-
Lymphotropic Viruses: Chronic Inflammation and Induction of Cancers.Biology (Basel). 2020 Nov 10;9(11):390. doi: 10.3390/biology9110390. Biology (Basel). 2020. PMID: 33182552 Free PMC article. Review.
-
Gambogic Acid as a Candidate for Cancer Therapy: A Review.Int J Nanomedicine. 2020 Dec 22;15:10385-10399. doi: 10.2147/IJN.S277645. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33376327 Free PMC article. Review.
-
Novel sorafenib analogues induce apoptosis through SHP-1 dependent STAT3 inactivation in human breast cancer cells.Breast Cancer Res. 2013;15(4):R63. doi: 10.1186/bcr3457. Breast Cancer Res. 2013. PMID: 23938089 Free PMC article.
References
-
- Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008;51:2589–2599. - PubMed
-
- Yang QZ, Jia SJ, DH L. The neoteric study of chinese traditional drug, gamboge. Chin J Clin Oncol. 1994;21:464–465.
-
- Zhu X, Zhang H, Lin Y, et al. Mechanisms of gambogic acid-induced apoptosis in non-small cell lung cancer cells in relation to transferrin receptors. J Chemother. 2009;21:666–672. - PubMed
-
- Mu R, Lu N, Wang J, et al. An oxidative analogue of gambogic acid-induced apoptosis of human hepatocellular carcinoma cell line HepG2 is involved in its anticancer activity in vitro. Eur J Cancer Prev. 2010;19:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

