AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila
- PMID: 21490149
- PMCID: PMC3113773
- DOI: 10.1091/mbc.E11-01-0054
AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila
Abstract
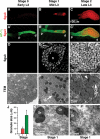
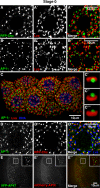
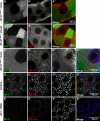
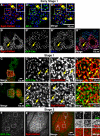
Regulated secretion of hormones, digestive enzymes, and other biologically active molecules requires the formation of secretory granules. Clathrin and the clathrin adaptor protein complex 1 (AP-1) are necessary for maturation of exocrine, endocrine, and neuroendocrine secretory granules. However, the initial steps of secretory granule biogenesis are only minimally understood. Powerful genetic approaches available in the fruit fly Drosophila melanogaster were used to investigate the molecular pathway for biogenesis of the mucin-containing "glue granules" that form within epithelial cells of the third-instar larval salivary gland. Clathrin and AP-1 colocalize at the trans-Golgi network (TGN) and clathrin recruitment requires AP-1. Furthermore, clathrin and AP-1 colocalize with secretory cargo at the TGN and on immature granules. Finally, loss of clathrin or AP-1 leads to a profound block in secretory granule formation. These findings establish a novel role for AP-1- and clathrin-dependent trafficking in the biogenesis of mucin-containing secretory granules.
Figures

Similar articles
-
Functional characterization of protein-sorting machineries at the trans-Golgi network in Drosophila melanogaster.J Cell Sci. 2010 Feb 1;123(Pt 3):460-71. doi: 10.1242/jcs.055103. Epub 2010 Jan 12. J Cell Sci. 2010. PMID: 20067992 Free PMC article.
-
AP-1A controls secretory granule biogenesis and trafficking of membrane secretory granule proteins.Traffic. 2014 Oct;15(10):1099-121. doi: 10.1111/tra.12194. Epub 2014 Aug 15. Traffic. 2014. PMID: 25040637 Free PMC article.
-
A novel function for Rab1 and Rab11 during secretory granule maturation.J Cell Sci. 2021 Aug 1;134(15):jcs259037. doi: 10.1242/jcs.259037. Epub 2021 Aug 3. J Cell Sci. 2021. PMID: 34342349 Free PMC article.
-
Trafficking/sorting and granule biogenesis in the beta-cell.Semin Cell Dev Biol. 2000 Aug;11(4):243-51. doi: 10.1006/scdb.2000.0173. Semin Cell Dev Biol. 2000. PMID: 10966858 Review.
-
Rediscovering the intricacies of secretory granule biogenesis.Curr Opin Cell Biol. 2023 Dec;85:102231. doi: 10.1016/j.ceb.2023.102231. Epub 2023 Aug 30. Curr Opin Cell Biol. 2023. PMID: 37657367 Review.
Cited by
-
Regulated Restructuring of Mucins During Secretory Granule Maturation In Vivo.Proc Natl Acad Sci U S A. 2022 Oct 25;119(43):e2209750119. doi: 10.1073/pnas.2209750119. Epub 2022 Oct 17. Proc Natl Acad Sci U S A. 2022. PMID: 36252017 Free PMC article.
-
A Rab6 to Rab11 transition is required for dense-core granule and exosome biogenesis in Drosophila secondary cells.PLoS Genet. 2023 Oct 16;19(10):e1010979. doi: 10.1371/journal.pgen.1010979. eCollection 2023 Oct. PLoS Genet. 2023. PMID: 37844085 Free PMC article.
-
Type II phosphatidylinositol 4-kinase regulates trafficking of secretory granule proteins in Drosophila.Development. 2012 Aug;139(16):3040-50. doi: 10.1242/dev.077644. Epub 2012 Jul 12. Development. 2012. PMID: 22791894 Free PMC article.
-
The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases.Traffic. 2019 Jun;20(6):404-435. doi: 10.1111/tra.12646. Traffic. 2019. PMID: 30945407 Free PMC article. Review.
-
Adherens Junctions on the Move-Membrane Trafficking of E-Cadherin.Cold Spring Harb Perspect Biol. 2017 Mar 1;9(3):a029140. doi: 10.1101/cshperspect.a029140. Cold Spring Harb Perspect Biol. 2017. PMID: 28096264 Free PMC article. Review.
References
-
- Abrams EW, Andrew DJ. CrebA regulates secretory activity in the Drosophila salivary gland and epidermis. Development. 2005;132:2743–2758. - PubMed
-
- Abrams EW, Mihoulides WK, Andrew DJ. Fork head and Sage maintain a uniform and patent salivary gland lumen through regulation of two downstream target genes, PH4αSG1 and PH4αSG2. Development. 2006;133:3517–3527. - PubMed
-
- Ashburner M. Drosophila: A Laboratory Handbook. Cold Spring Harbor, NY:: Cold Spring Harbor Laboratory; 1990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

