The long-term memory trace formed in the Drosophila α/β mushroom body neurons is abolished in long-term memory mutants
- PMID: 21490205
- PMCID: PMC3118425
- DOI: 10.1523/JNEUROSCI.3190-10.2011
The long-term memory trace formed in the Drosophila α/β mushroom body neurons is abolished in long-term memory mutants
Abstract
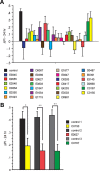
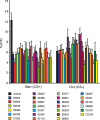
A prior screen identified dozens of Drosophila melanogaster mutants that possess defective long-term memory (LTM). Using spaced olfactory conditioning, we trained 26 of these mutant lines to associate an odor cue with electric shock and then examined the memory of this conditioning 24 h later. All of the mutants tested revealed a deficit in LTM compared to the robust LTM observed in control flies. We used in vivo functional optical imaging to measure the magnitude of a previously characterized LTM trace, which is manifested as increased calcium influx into the axons of α/β mushroom body neurons in response to the conditioned odor. This memory trace was defective in all 26 of the LTM mutants. These observations elevate the significance of this LTM trace given that 26 independent mutants all exhibit a defect in the trace, and further suggest that the calcium trace is a fundamental mechanism underlying Drosophila LTM.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
