Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7
- PMID: 21490207
- PMCID: PMC6622839
- DOI: 10.1523/JNEUROSCI.6638-10.2011
Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7
Abstract
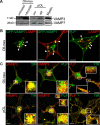
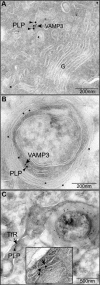
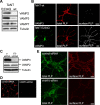
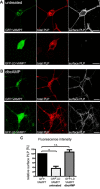
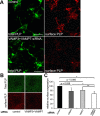
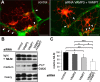
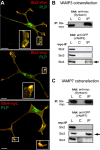
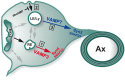
CNS myelination by oligodendrocytes requires directed transport of myelin membrane components and a timely and spatially controlled membrane expansion. In this study, we show the functional involvement of the R-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (R-SNARE) proteins VAMP3/cellubrevin and VAMP7/TI-VAMP in myelin membrane trafficking. VAMP3 and VAMP7 colocalize with the major myelin proteolipid protein (PLP) in recycling endosomes and late endosomes/lysosomes, respectively. Interference with VAMP3 or VAMP7 function using small interfering RNA-mediated silencing and exogenous expression of dominant-negative proteins diminished transport of PLP to the oligodendroglial cell surface. In addition, the association of PLP with myelin-like membranes produced by oligodendrocytes cocultured with cortical neurons was reduced. We furthermore identified Syntaxin-4 and Syntaxin-3 as prime acceptor Q-SNAREs of VAMP3 and VAMP7, respectively. Analysis of VAMP3-deficient mice revealed no myelination defects. Interestingly, AP-3δ-deficient mocha mice, which suffer from impaired secretion of lysosome-related organelles and missorting of VAMP7, exhibit a mild dysmyelination characterized by reduced levels of select myelin proteins, including PLP. We conclude that PLP reaches the cell surface via at least two trafficking pathways with distinct regulations: (1) VAMP3 mediates fusion of recycling endosome-derived vesicles with the oligodendroglial plasma membrane in the course of the secretory pathway; (2) VAMP7 controls exocytosis of PLP from late endosomal/lysosomal organelles as part of a transcytosis pathway. Our in vivo data suggest that exocytosis of lysosome-related organelles controlled by VAMP7 contributes to myelin biogenesis by delivering cargo to the myelin membrane.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
