Transcription factor Foxd1 is required for the specification of the temporal retina in mammals
- PMID: 21490208
- PMCID: PMC6622810
- DOI: 10.1523/JNEUROSCI.0394-11.2011
Transcription factor Foxd1 is required for the specification of the temporal retina in mammals
Abstract
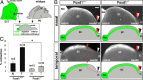
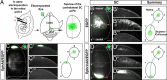
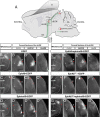
The organization of the visual system is different in birds and mammals. In both, retinal axons project topographically to the visual targets in the brain; but whereas in birds visual fibers from the entire retina decussate at the optic chiasm, in mammals, a number of axons from the temporal retina diverge at the midline to project ipsilaterally. Gain-of-function experiments in chick raised the hypothesis that the transcription factor Foxd1 specifies retinal temporal identity. However, it remains unknown whether Foxd1 is necessary for this function. In mammals, the crucial role of Foxd1 in the patterning of the optic chiasm region has complicated the interpretation of its cell-autonomous function in the retina. Furthermore, target molecules identified for Foxd1 are different in chicks and mice, leading to question the function of Foxd1 in mammals. Here we show that in the mouse, Foxd1 imprints temporal features in the retina such as axonal ipsilaterality and rostral targeting in collicular areas and that EphA6 is a Foxd1 downstream effector that sends temporal axons to the rostral colliculus. In addition, our data support a model in which the desensitization of EphA6 by ephrinA5 in cis is not necessary for the proper functioning of EphA6. Overall, these results indicate that Foxd1 functions as a conserved determinant of temporal identity but reveal that the downstream effectors, and likely their mechanisms of action, are different in mammals and birds.
Figures




References
-
- Brown A, Yates PA, Burrola P, Ortuno D, Vaidya A, Jessell TM, Pfaff SL, O'Leary DD, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102:77–88. - PubMed
-
- Brown LY, Kottman AH, Brown S. Immunolocalization of zic2 expression in the developing forebrain. Gene Expr Patterns. 2003;3:361–367. - PubMed
-
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
