Recovery from short-term depression and facilitation is ultrafast and Ca2+ dependent at auditory hair cell synapses
- PMID: 21490209
- PMCID: PMC3090423
- DOI: 10.1523/JNEUROSCI.5453-10.2011
Recovery from short-term depression and facilitation is ultrafast and Ca2+ dependent at auditory hair cell synapses
Abstract
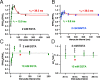
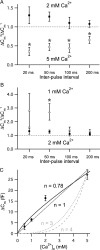
Short-term facilitation and depression coexist at many CNS synapses. Facilitation, however, has not been fully characterized at hair cell synapses. Using paired recordings and membrane capacitance measurements we find that paired-pulse plasticity at an adult frog auditory hair cell synapse depends on pulse duration and interpulse intervals. For short 20 ms depolarizing pulses, and interpulse intervals between 15 and 50 ms, facilitation occurred when hair cells were held at -90 mV. However, hair cells held at -60 mV displayed only paired-pulse depression. Facilitation was dependent on residual free Ca2+ levels because it was greatly reduced by the Ca2+ buffers EGTA and BAPTA. Furthermore, low external Ca2+ augmented facilitation, whereas depression was augmented by high external Ca2+, consistent with depletion of a small pool of fast releasing synaptic vesicles. Recovery from depression had a double-exponential time course with a fast component that may reflect the rapid replenishment of a depleted vesicle pool. We suggest that hair cells held at more depolarized in vivo-like resting membrane potentials have a tonic influx of Ca2+; they are thus in a dynamic state of continuous vesicle release, pool depletion and replenishment. Further Ca2+ influx during paired-pulse stimuli then leads to depression. However, at membrane potentials of -90 mV, ongoing release and pool depletion are minimized, so facilitation is revealed at time intervals when rapid vesicle pool replenishment occurs. Finally, we propose that vesicle pool replenishment kinetics is not rate limited by vesicle endocytosis, which is too slow to influence the rapid pool replenishment process.
Figures







References
-
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
