Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice
- PMID: 21490217
- PMCID: PMC3135337
- DOI: 10.1523/JNEUROSCI.5346-10.2011
Enhanced subcortical spreading depression in familial hemiplegic migraine type 1 mutant mice
Abstract
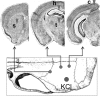
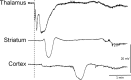
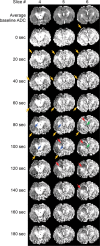
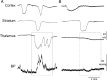
Familial hemiplegic migraine type 1, a monogenic migraine variant with aura, is linked to gain-of-function mutations in the CACNA1A gene encoding Ca(V)2.1 channels. The S218L mutation causes severe channel dysfunction, and paroxysmal migraine attacks can be accompanied by seizures, coma, and hemiplegia; patients expressing the R192Q mutation exhibit hemiplegia only. Familial hemiplegic migraine knock-in mice expressing the S218L or R192Q mutation are highly susceptible to cortical spreading depression, the electrophysiological surrogate for migraine aura, and develop severe and prolonged motor deficits after spreading depression. The S218L mutants also develop coma and seizures and sometimes die. To investigate underlying mechanisms for these symptoms, we used multielectrode electrophysiological recordings, diffusion-weighted magnetic resonance imaging, and c-fos immunohistochemistry to trace spreading depression propagation into subcortical structures. We showed that unlike the wild type, cortical spreading depression readily propagated into subcortical structures in both familial hemiplegic migraine type 1 mutants. Whereas the facilitated subcortical spread appeared limited to the striatum in R192Q, hippocampal and thalamic spread was detected in the S218L mutants with an allele-dosage effect. Both strains exhibited increased susceptibility to subcortical spreading depression and reverberating spreading depression waves. Altogether, these data show that spreading depression propagates between cortex, basal ganglia, diencephalon, and hippocampus in genetically susceptible brains, which could explain the prolonged hemiplegia, coma, and seizure phenotype in this variant of migraine with aura.
Figures



References
-
- Avoli M, Drapeau C, Louvel J, Pumain R, Olivier A, Villemure JG. Epileptiform activity induced by low extracellular magnesium in the human cortex maintained in vitro. Ann Neurol. 1991;30:589–596. - PubMed
-
- Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. - PubMed
-
- Battistini S, Stenirri S, Piatti M, Gelfi C, Righetti PG, Rocchi R, Giannini F, Battistini N, Guazzi GC, Ferrari M, Carrera P. A new CACNA1A gene mutation in acetazolamide-responsive familial hemiplegic migraine and ataxia. Neurology. 1999;53:38–43. - PubMed
-
- Bures J, Fifkova E. The effect of potassium ions liberated from nerve structures invaded by spreading depression on conduction in fibres of the adjacent white matter. Physiol Bohemoslov. 1968;17:405–410. - PubMed
-
- Bures J, Hartman G. Conduction block in capsula interna fibres caused by striatal spreading depression in rats. Experientia. 1967;23:736–737. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
