The Down syndrome critical region regulates retinogeniculate refinement
- PMID: 21490218
- PMCID: PMC3230532
- DOI: 10.1523/JNEUROSCI.6015-10.2011
The Down syndrome critical region regulates retinogeniculate refinement
Abstract
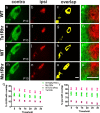
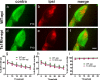
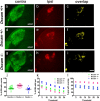
Down syndrome (DS) is a developmental disorder caused by a third chromosome 21 in humans (Trisomy 21), leading to neurological deficits and cognitive impairment. Studies in mouse models of DS suggest that cognitive deficits in the adult are associated with deficits in synaptic learning and memory mechanisms, but it is unclear whether alterations in the early wiring and refinement of neuronal circuits contribute to these deficits. Here, we show that early developmental refinement of visual circuits is perturbed in mouse models of Down syndrome. Specifically, we find excessive eye-specific segregation of retinal axons in the dorsal lateral geniculate nucleus. Indeed, the degree of refinement scales with defects in the "Down syndrome critical region" (DSCR) in a dose-dependent manner. We further identify Dscam (Down syndrome cell adhesion molecule), a gene within the DSCR, as a regulator of eye-specific segregation of retinogeniculate projections. Although Dscam is not the sole gene in the DSCR contributing to enhanced refinement in trisomy, Dscam dosage clearly regulates cell spacing and dendritic fasciculation in a specific class of retinal ganglion cells. Thus, altered developmental refinement of visual circuits that occurs before sensory experience is likely to contribute to visual impairment in individuals with Down syndrome.
Figures






References
-
- Barlow GM, Chen XN, Shi ZY, Lyons GE, Kurnit DM, Celle L, Spinner NB, Zackai E, Pettenati MJ, Van Riper AJ, Vekemans MJ, Mjaatvedt CH, Korenberg JR. Down syndrome congenital heart disease: a narrowed region and a candidate gene. Genet Med. 2001;3:91–101. - PubMed
-
- Barlow GM, Micales B, Chen XN, Lyons GE, Korenberg JR. Mammalian DSCAMs: roles in the development of the spinal cord, cortex, and cerebellum? Biochem Biophys Res Commun. 2002;293:881–891. - PubMed
-
- Becker LE, Armstrong DL, Chan F. Dendritic atrophy in children with Down's syndrome. Ann Neurol. 1986;20:520–526. - PubMed
-
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
