Somatic depolarization enhances GABA release in cerebellar interneurons via a calcium/protein kinase C pathway
- PMID: 21490222
- PMCID: PMC6622834
- DOI: 10.1523/JNEUROSCI.5127-10.2011
Somatic depolarization enhances GABA release in cerebellar interneurons via a calcium/protein kinase C pathway
Abstract
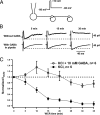
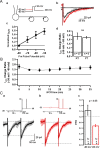
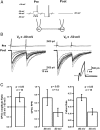
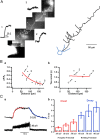
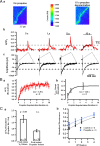
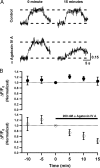
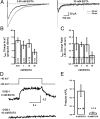
In cortical and hippocampal neurons, tonic somatic depolarization is partially transmitted to synaptic terminals, where it enhances transmitter release. It is not known to what extent such "analog signaling" applies to other mammalian neurons, and available evidence concerning underlying mechanisms is fragmentary and partially controversial. In this work, we investigate the presence of analog signaling in molecular layer interneurons of the rat cerebellum. GABA release was estimated by measuring autoreceptor currents in single recordings, or postsynaptic currents in paired recordings of synaptically connected neurons. We find with both assays that moderate subthreshold somatic depolarization results in enhanced GABA release. In addition, changes in the calcium concentration were investigated in the axon compartment using the calcium-sensitive dye OGB-1 (Oregon Green BAPTA-1). After a step somatic depolarization, the axonal calcium concentration and the GABA release probability rise with a common slow time course. However, the amount of calcium entry that is associated to one action potential is not affected. The slow increase in calcium concentration is inhibited by the P/Q calcium channel blocker ω-agatoxin-IVA. The protein kinase C inhibitor Ro 31-8220 (3-[3-[2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl]-1H-indol-1-yl]propyl carbamimidothioic acid ester mesylate) did not affect the calcium concentration changes but it blocked the increase in GABA release. EGTA was a weak blocker of analog signaling, implicating a close association of protein kinase C to the site of calcium entry. We conclude that analog signaling is prominent in cerebellar interneurons and that it is triggered by a pathway involving activation of axonal P/Q channels, followed by calcium entry and local activation of protein kinase C.
Figures

References
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr Opin Neurobiol. 2008;18:314–320. - PubMed
-
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. - PubMed
-
- Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003;6:551–552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
