Body weight-dependent troponin T alternative splicing is evolutionarily conserved from insects to mammals and is partially impaired in skeletal muscle of obese rats
- PMID: 21490260
- PMCID: PMC3076076
- DOI: 10.1242/jeb.051763
Body weight-dependent troponin T alternative splicing is evolutionarily conserved from insects to mammals and is partially impaired in skeletal muscle of obese rats
Abstract
Do animals know at a physiological level how much they weigh, and, if so, do they make homeostatic adjustments in response to changes in body weight? Skeletal muscle is a likely tissue for such plasticity, as weight-bearing muscles receive mechanical feedback regarding body weight and consume ATP in order to generate forces sufficient to counteract gravity. Using rats, we examined how variation in body weight affected alternative splicing of fast skeletal muscle troponin T (Tnnt3), a component of the thin filament that regulates the actin-myosin interaction during contraction and modulates force output. In response to normal growth and experimental body weight increases, alternative splicing of Tnnt3 in rat gastrocnemius muscle was adjusted in a quantitative fashion. The response depended on weight per se, as externally attached loads had the same effect as an equal change in actual body weight. Examining the association between Tnnt3 alternative splicing and ATP consumption rate, we found that the Tnnt3 splice form profile had a significant association with nocturnal energy expenditure, independently of effects of weight. For a subset of the Tnnt3 splice forms, obese Zucker rats failed to make the same adjustments; that is, they did not show the same relationship between body weight and the relative abundance of five Tnnt3 β splice forms (i.e. Tnnt3 β2-β5 and β8), four of which showed significant effects on nocturnal energy expenditure in Sprague-Dawley rats. Heavier obese Zucker rats displayed certain splice form relative abundances (e.g. Tnnt3 β3) characteristic of much lighter, lean animals, resulting in a mismatch between body weight and muscle molecular composition. Consequently, we suggest that body weight-inappropriate skeletal muscle Tnnt3 expression in obesity is a candidate mechanism for muscle weakness and reduced mobility. Weight-dependent quantitative variation in Tnnt3 alternative splicing appears to be an evolutionarily conserved feature of skeletal muscle and provides a quantitative molecular marker to track how an animal perceives and responds to body weight.
Figures

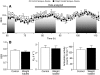
 ) as a function of time for weight-loaded and control rats maintained in indirect calorimetry chambers for 5 days, during the last ∼50 h of the experiment (N=16 rats). Shaded areas indicate the dark period of the 12 h light/dark cycle. Statistical analyses of the effects of weight loading on energy expenditure and activity (see also Table 3) were performed on data obtained during the last full day (i.e. animals had been weight loaded for 4 days). (B) Comparison of mean RER (0.88±0.009 vs 0.86±0.01), mean
) as a function of time for weight-loaded and control rats maintained in indirect calorimetry chambers for 5 days, during the last ∼50 h of the experiment (N=16 rats). Shaded areas indicate the dark period of the 12 h light/dark cycle. Statistical analyses of the effects of weight loading on energy expenditure and activity (see also Table 3) were performed on data obtained during the last full day (i.e. animals had been weight loaded for 4 days). (B) Comparison of mean RER (0.88±0.009 vs 0.86±0.01), mean  (2589.3±312.2 vs 2514.2±310.2 ml h–1) and mean ambulatory activity (139.2±13.8 vs 150.2±18.5 counts 15 min–1) during day 4. No differences were detected between weight-loaded and control animals. Means are presented with s.e.m. (error bars).
(2589.3±312.2 vs 2514.2±310.2 ml h–1) and mean ambulatory activity (139.2±13.8 vs 150.2±18.5 counts 15 min–1) during day 4. No differences were detected between weight-loaded and control animals. Means are presented with s.e.m. (error bars).


Similar articles
-
Cell-autonomous regulation of fast troponin T pre-mRNA alternative splicing in response to mechanical stretch.Am J Physiol Cell Physiol. 2012 Aug 1;303(3):C298-307. doi: 10.1152/ajpcell.00400.2011. Epub 2012 May 16. Am J Physiol Cell Physiol. 2012. PMID: 22592404 Free PMC article.
-
Effects of age and hindlimb immobilization and remobilization on fast troponin T precursor mRNA alternative splicing in rat gastrocnemius muscle.Appl Physiol Nutr Metab. 2016 Feb;41(2):142-9. doi: 10.1139/apnm-2015-0381. Epub 2015 Oct 16. Appl Physiol Nutr Metab. 2016. PMID: 26799695 Free PMC article.
-
Dietary Fat Quantity and Type Induce Transcriptome-Wide Effects on Alternative Splicing of Pre-mRNA in Rat Skeletal Muscle.J Nutr. 2017 Sep;147(9):1648-1657. doi: 10.3945/jn.117.254482. Epub 2017 Aug 2. J Nutr. 2017. PMID: 28768832 Free PMC article.
-
TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships.Gene. 2016 May 10;582(1):1-13. doi: 10.1016/j.gene.2016.01.006. Epub 2016 Jan 13. Gene. 2016. PMID: 26774798 Free PMC article. Review.
-
Thin filament proteins mutations associated with skeletal myopathies: defective regulation of muscle contraction.J Mol Med (Berl). 2008 Nov;86(11):1197-204. doi: 10.1007/s00109-008-0380-9. Epub 2008 Jun 24. J Mol Med (Berl). 2008. PMID: 18574571 Review.
Cited by
-
Cell-autonomous regulation of fast troponin T pre-mRNA alternative splicing in response to mechanical stretch.Am J Physiol Cell Physiol. 2012 Aug 1;303(3):C298-307. doi: 10.1152/ajpcell.00400.2011. Epub 2012 May 16. Am J Physiol Cell Physiol. 2012. PMID: 22592404 Free PMC article.
-
Effects of age and hindlimb immobilization and remobilization on fast troponin T precursor mRNA alternative splicing in rat gastrocnemius muscle.Appl Physiol Nutr Metab. 2016 Feb;41(2):142-9. doi: 10.1139/apnm-2015-0381. Epub 2015 Oct 16. Appl Physiol Nutr Metab. 2016. PMID: 26799695 Free PMC article.
-
Influence of ageing and essential amino acids on quantitative patterns of troponin T alternative splicing in human skeletal muscle.Appl Physiol Nutr Metab. 2015 Aug;40(8):788-796. doi: 10.1139/apnm-2014-0568. Epub 2015 Mar 23. Appl Physiol Nutr Metab. 2015. PMID: 26201856 Free PMC article.
-
Integrative Analysis of Nanopore and Illumina Sequencing Reveals Alternative Splicing Complexity in Pig Longissimus Dorsi Muscle.Front Genet. 2022 Apr 11;13:877646. doi: 10.3389/fgene.2022.877646. eCollection 2022. Front Genet. 2022. PMID: 35480309 Free PMC article.
-
Influence of weighted downhill running training on serial sarcomere number and work loop performance in the rat soleus.Biol Open. 2022 Jul 15;11(7):bio059491. doi: 10.1242/bio.059491. Epub 2022 Jul 25. Biol Open. 2022. PMID: 35876382 Free PMC article.
References
-
- Anandacoomarasamy A., Caterson I., Sambrook P., Fransen M., March L. (2008). The impact of obesity on the musculoskeletal system. Int. J. Obes. (Lond.) 32, 2112-2122 - PubMed
-
- Anthony J. C., Yoshizawa F., Anthony T. G., Vary T. C., Jefferson L. S., Kimball S. R. (2000). Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 130, 2413-2419 - PubMed
-
- Biewener A. A. (1989). Scaling body support in mammals: limb posture and muscle mechanics. Science 245, 45-48 - PubMed
-
- Blaustein M., Pelisch F., Tanos T., Muñoz M. J., Wengier D., Quadrana L., Sanford J. R., Muschietti J. P., Kornblihtt A. R., Cáceres J. F., et al. (2005). Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat. Struct. Mol. Biol. 12, 1037-1044 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

