Short-term modulation at synapses between neurons in laminae II-V of the rodent spinal dorsal horn
- PMID: 21490280
- PMCID: PMC3118754
- DOI: 10.1152/jn.00684.2010
Short-term modulation at synapses between neurons in laminae II-V of the rodent spinal dorsal horn
Abstract
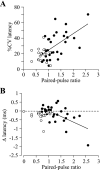
Unitary excitatory (EPSP) and inhibitory (IPSP) postsynaptic potentials (PSPs) were evoked between neurons in Rexed's laminae (L)II-V of spinal slices from young hamsters (7-24 days old) at 27°C using paired whole cell recordings. Laminar differences in synaptic efficacy were observed: excitatory connections were more secure than inhibitory connections in LII and inhibitory linkages in LII were less reliable than those in LIII-V. A majority of connections displayed paired-pulse facilitation or depression. Depression was observed for both EPSPs and IPSPs, but facilitation was seen almost exclusively for IPSPs. There were no frequency-dependent shifts between facilitation and depression. Synaptic depression was associated with an increased failure rate and decreased PSP half-width for a majority of connections. However, there were no consistent changes in failure rate or PSP time course at facilitating connections. IPSPs evoked at high-failure synapses had consistently smaller amplitude and showed greater facilitation than low-failure connections. Facilitation at inhibitory connections was positively correlated with synaptic jitter and associated with a decrease in latency. At many connections, the paired-pulse ratio varied from trial to trial and depended on the amplitude of the first PSP; dependence was greater for inhibitory synapses than excitatory synapses. Paired-pulse ratios for connections onto neurons with rapidly adapting, "phasic" discharge to depolarizing current injection were significantly greater than for connections onto neurons with tonic discharge properties. These results are evidence of diversity in synaptic transmission between dorsal horn neurons, the nature of which may depend on the types of linkage, laminar location, and intrinsic firing properties of postsynaptic cells.
Figures

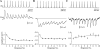


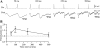

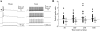
Similar articles
-
Local circuit connections between hamster laminae III and IV dorsal horn neurons.J Neurophysiol. 2008 Mar;99(3):1306-18. doi: 10.1152/jn.00962.2007. Epub 2008 Jan 9. J Neurophysiol. 2008. PMID: 18184889
-
Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III-IV of the young rat spinal cord.Eur J Neurosci. 2007 Nov;26(10):2940-9. doi: 10.1111/j.1460-9568.2007.05919.x. Eur J Neurosci. 2007. PMID: 18001289
-
Spatiotemporal patterns of dorsal root-evoked network activity in the neonatal rat spinal cord: optical and intracellular recordings.J Neurophysiol. 2005 Sep;94(3):1952-61. doi: 10.1152/jn.00209.2005. Epub 2005 May 11. J Neurophysiol. 2005. PMID: 15888530
-
Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn.Prog Neurobiol. 1993 Aug;41(2):125-56. doi: 10.1016/0301-0082(93)90006-e. Prog Neurobiol. 1993. PMID: 8392739 Review. No abstract available.
-
Shared and divergent principles of synaptic transmission between cortical excitatory neurons in rodent and human brain.Front Synaptic Neurosci. 2023 Sep 5;15:1274383. doi: 10.3389/fnsyn.2023.1274383. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 37731775 Free PMC article. Review.
Cited by
-
Pain processing by spinal microcircuits: afferent combinatorics.Curr Opin Neurobiol. 2012 Aug;22(4):631-9. doi: 10.1016/j.conb.2012.02.010. Epub 2012 Mar 10. Curr Opin Neurobiol. 2012. PMID: 22409855 Free PMC article.
-
Three-dimensional organization of local excitatory and inhibitory inputs to neurons in laminae III-IV of the spinal dorsal horn.J Physiol. 2013 Nov 15;591(22):5645-60. doi: 10.1113/jphysiol.2013.256016. Epub 2013 Aug 27. J Physiol. 2013. PMID: 23981716 Free PMC article.
-
Subpopulation-specific patterns of intrinsic connectivity in mouse superficial dorsal horn as revealed by laser scanning photostimulation.J Physiol. 2013 Apr 1;591(7):1935-49. doi: 10.1113/jphysiol.2012.244210. Epub 2013 Jan 7. J Physiol. 2013. PMID: 23297304 Free PMC article.
-
Cav3.2 T-type calcium channels shape electrical firing in mouse Lamina II neurons.Sci Rep. 2019 Feb 28;9(1):3112. doi: 10.1038/s41598-019-39703-3. Sci Rep. 2019. PMID: 30816223 Free PMC article.
References
-
- Angulo MC, Staiger JF, Rossier J, Audinat E. Distinct local circuits between neocortical pyramidal cells and fast-spiking interneurons in young adult rats. J Neurophysiol 89: 943–953, 2003 - PubMed
-
- Beal JA, Russell CT, Knight DS. Morphological and developmental characterization of local-circuit neurons in lamina III of the rat spinal cord. Neurosci Lett 86: 1–5, 1988 - PubMed
-
- Bicknell HR, Beal JA. Axonal and dendritic development of substantia gelatinosa neurons in the lumbosacral spinal cord of the rat. J Comp Neurol 226: 508–522, 1984 - PubMed
-
- Cabezas C, Buño W. Distinct transmitter release properties determine differences in short-term plasticity at functional and silent synapses. J Neurophysiol 95: 3024–3034, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

