Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle
- PMID: 21490328
- PMCID: PMC3129914
- DOI: 10.1152/ajpheart.01020.2010
Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle
Abstract
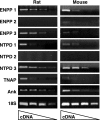
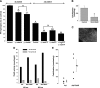
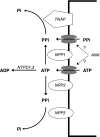
Extracellular inorganic pyrophosphate (ePP(i)) is an important endogenous inhibitor of vascular calcification, but it is not known whether systemic or local vascular PP(i) metabolism controls calcification. To determine the role of ePP(i) in vascular smooth muscle, we identified the pathways responsible for ePP(i) production and hydrolysis in rat and mouse aortas and manipulated them to demonstrate their role in the calcification of isolated aortas in culture. Rat and mouse aortas contained mRNA for ectonucleotide pyrophosphatase/phosphodiesterases (NPP1-3), the putative PP(i) transporter ANK, and tissue-nonspecific alkaline phosphatase (TNAP). Synthesis of PP(i) from ATP in aortas was blocked by β,γ-methylene-ATP, an inhibitor of NPPs. Aortas from mice lacking NPP1 (Enpp1(-/-)) did not synthesize PP(i) from ATP and exhibited increased calcification in culture. Although ANK-mediated transport of PP(i) could not be demonstrated in aortas, aortas from mutant (ank/ank) mice calcified more in culture than did aortas from normal (ANK/ANK) mice. Hydrolysis of PP(i) was reduced 25% by β,γ-methylene-ATP and 50% by inhibition of TNAP. Hydrolysis of PP(i) was increased in cells overexpressing TNAP or NPP3 but not NPP1 and was not reduced in Enpp1(-/-) aortas. Overexpression of TNAP increased calcification of cultured aortas. The results show that smooth muscle NPP1 and TNAP control vascular calcification through effects on synthesis and hydrolysis of ePP(i), indicating an important inhibitory role of locally produced PP(i). Smooth muscle ANK also affects calcification, but this may not be mediated through transport of PP(i). NPP3 is identified as an additional pyrophosphatase that could influence vascular calcification.
Figures

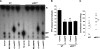
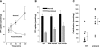
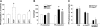
References
-
- Costello JC, Rosenthal AK, Kurup IV, Masuda I, Medhora M, Ryan LM. Parallel regulation of extracellular ATP and inorganic pyrophosphate: roles of growth factors, transduction modulators, and ANK. Connect Tissue Res 52: 139–146, 2011 - PubMed
-
- Dahl R, Sergienko EA, Su Y, Mostofi YS, Yang L, Simao AM, Narisawa S, Brown B, Mangravita-Novo A, Vicchiarelli M, Smith LH, O′Neill WC, Millan JL, Cosford ND. Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). J Med Chem 52: 6919–6925, 2009 - PMC - PubMed
-
- Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289: 265–270, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

