Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy
- PMID: 21490404
- PMCID: PMC3069769
- DOI: 10.1172/JCI44145
Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy
Abstract
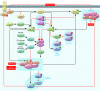
Mammalian target of rapamycin (mTOR) is a PI3K-related kinase that regulates cell growth, proliferation, and survival via mTOR complex 1 (mTORC1) and mTORC2. The mTOR pathway is often aberrantly activated in cancers. While hypoxia, nutrient deprivation, and DNA damage restrain mTORC1 activity, multiple genetic events constitutively activate mTOR in cancers. Here we provide a brief overview of the signaling pathways up- and downstream of mTORC1 and -2, and discuss the insights into therapeutic anticancer targets - both those that have been tried in the clinic with limited success and those currently under clinical development - that knowledge of these pathways gives us.
Figures
References
-
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975;28(10):721–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

