Trask phosphorylation defines the reverse mode of a phosphotyrosine signaling switch that underlies cell anchorage state
- PMID: 21490433
- PMCID: PMC3117134
- DOI: 10.4161/cc.10.8.15343
Trask phosphorylation defines the reverse mode of a phosphotyrosine signaling switch that underlies cell anchorage state
Abstract
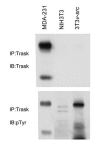
Phosphotyrosine signaling in anchored epithelial cells constitutes a spacially ordained signaling program that largely functions to promote integrin-linked focal adhesion complexes, serving to secure cell anchorage to matrix and as a bidirectional signaling hub that coordinates the physical state of the cell and its environment with cellular functions including proliferation and survival. Cells release their adhesions during processes such as mitosis, migration, or tumorigenesis, but the fate of signaling through tyrosine phosphorylation in unanchored cells remains poorly understood. In an examination of epithelial cells in the unanchored state, we find abundant phosphotyrosine signaling, largely recommitted to an anti-adhesive function mediated through the Src family phosphorylation of their transmembrane substrate Trask/CDCP1/gp140. Src-Trask phosphorylation inhibits integrin clustering and focal adhesion assembly and signaling, defining an active phosphotyrosine signaling program underlying the unanchored state. Src-Trask signaling and Src-focal adhesion signaling inactivate each other, constituting two opposing modes of phosphotyrosine signaling that define a switch underline cell anchorage state. Src kinases are prominent drivers of both signaling modes, identifying their position at the helm of adhesion signaling capable of specifying anchorage state through substrate selection. These experimental studies along with concurring phylogenetic evidence suggest that phosphorylation on tyrosine is a signaling function fundamentally linked with the regulation of integrins.
Figures




References
-
- Vuori K. Integrin signaling: Tyrosine phosphorylation events in focal adhesions. J Membr Biol. 1998;165:191–199. - PubMed
-
- Kirchner J, Kam Z, Tzur G, Bershadsky AD, Geiger B. Live-cell monitoring of tyrosine phosphorylation in focal adhesions following microtubule disruption. J Cell Sci. 2003;116:975–986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
