MDCK cystogenesis driven by cell stabilization within computational analogues
- PMID: 21490722
- PMCID: PMC3072361
- DOI: 10.1371/journal.pcbi.1002030
MDCK cystogenesis driven by cell stabilization within computational analogues
Abstract
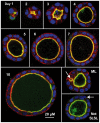
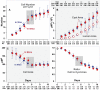
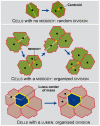
The study of epithelial morphogenesis is fundamental to increasing our understanding of organ function and disease. Great progress has been made through study of culture systems such as Madin-Darby canine kidney (MDCK) cells, but many aspects of even simple morphogenesis remain unclear. For example, are specific cell actions tightly coupled to the characteristics of the cell's environment or are they more often cell state dependent? How does the single lumen, single cell layer cyst consistently emerge from a variety of cell actions? To improve insight, we instantiated in silico analogues that used hypothesized cell behavior mechanisms to mimic MDCK cystogenesis. We tested them through in vitro experimentation and quantitative validation. We observed novel growth patterns, including a cell behavior shift that began around day five of growth. We created agent-oriented analogues that used the cellular Potts model along with an Iterative Refinement protocol. Following several refinements, we achieved a degree of validation for two separate mechanisms. Both survived falsification and achieved prespecified measures of similarity to cell culture properties. In silico components and mechanisms mapped to in vitro counterparts. In silico, the axis of cell division significantly affects lumen number without changing cell number or cyst size. Reducing the amount of in silico luminal cell death had limited effect on cystogenesis. Simulations provide an observable theory for cystogenesis based on hypothesized, cell-level operating principles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









References
-
- Gin E, Tanaka EM, Brusch L. A model for cyst lumen expansion and size regulation via fluid secretion. J Theor Biol. 2010;264:1077–1088. - PubMed
-
- Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95:137–151. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

