Lipid bilayer composition affects transmembrane protein orientation and function
- PMID: 21490797
- PMCID: PMC3068514
- DOI: 10.1155/2011/208457
Lipid bilayer composition affects transmembrane protein orientation and function
Abstract
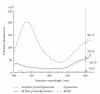
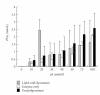
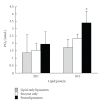
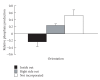
Sperm membranes change in structure and composition upon ejaculation to undergo capacitation, a molecular transformation which enables spermatozoa to undergo the acrosome reaction and be capable of fertilization. Changes to the membrane environment including lipid composition, specifically lipid microdomains, may be responsible for enabling capacitation. To study the effect of lipid environment on proteins, liposomes were created using lipids extracted from bull sperm membranes, with or without a protein (Na(+) K(+)-ATPase or α-amylase). Protein incorporation, function, and orientation were determined. Fluorescence resonance energy transfer (FRET) confirmed protein inclusion in the lipid bilayer, and protein function was confirmed using a colourometric assay of phosphate production from ATP cleavage. In the native lipid liposomes, ATPase was oriented with the β subunit facing the outer leaflet, while changing the lipid composition to 50% native lipids and 50% exogenous lipids significantly altered this orientation of Na(+) K(+)-ATPase within the membranes.
Figures


References
-
- Post H, Schwarz A, Brandenburger T, Aumüller G, Wilhelm B. Arrangement of PMCA4 in bovine sperm membrane fractions. International Journal of Andrology. 2010;33(6):775–783. - PubMed
-
- Edidin M. Lipids on the frontier: a century of cell-membrane bilayers. Nature Reviews Molecular Cell Biology. 2003;4(5):414–418. - PubMed
-
- Khalil MB, Chakrabandhu K, Xu H, et al. Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Developmental Biology. 2006;290(1):220–235. - PubMed
-
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Vol. 1. New York, NY, USA: Raven Press; 1994. pp. 189–317.
-
- Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganisation during capacitation. International Journal of Developmental Biology. 2008;52(5-6):473–480. - PubMed
LinkOut - more resources
Full Text Sources

