Regulation of Serum Response Factor and Adiponectin by PPARγ Agonist Docosahexaenoic Acid
- PMID: 21490806
- PMCID: PMC3066850
- DOI: 10.1155/2011/670479
Regulation of Serum Response Factor and Adiponectin by PPARγ Agonist Docosahexaenoic Acid
Abstract
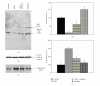
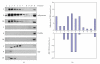
Recent studies indicate that significant health benefits involving the regulation of signaling proteins result from the consumption of omega-3 polyunsaturated fatty acids (ω-3 PUFAs). Serum response factor (SRF) is involved in transcriptional regulation of muscle growth and differentiation. SRF levels are increased in the aging heart muscle. It has not been examined whether SRF is made by adipocytes and whether SRF secretion by adipocytes is modulated by PPARγ agonist DHA. Adiponectin is made exclusively by adipocytes. We and others have previously reported that PUFAs such as DHA increase adiponectin levels and secretion in adipocytes. Here we show that DHA downregulates SRF with a simultaneous upregulation of adiponectin and that both these responses are reversible by PPARγ antagonist. Furthermore, there appears to be a direct relationship between DHA exposure and increased levels of membrane-associated high-density adiponectin, as well as lower levels of membrane-associated SRF. Thus, we find that the levels of SRF and adiponectin are inversely related in response to treatment with PPARγ agonist DHA. Decreased levels of SRF along with increase in membrane-associated adiponectin could in part mediate the health benefits of DHA.
Figures

References
-
- Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? American Journal of Medicine. 2007;120(9):S10–S16. - PubMed
-
- Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends in Cell Biology. 2006;16(11):588–596. - PubMed
-
- Pipes GCT, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes and Development. 2006;20(12):1545–1556. - PubMed
-
- Zhang X, Azhar G, Chai J, et al. Cardiomyopathy in transgenic mice with cardiac-specific overexpression of serum response factor. American Journal of Physiology—Heart and Circulatory Physiology. 2001;280(4):H1782–H1792. - PubMed
-
- Parlakian A, Charvet C, Escoubet B, et al. Temporally controlled onset of dilated cardiomyopathy through disruption of the srf gene in adult heart. Circulation. 2005;112(19):2930–2939. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

