The antioxidant effect of erythropoietin on thalassemic blood cells
- PMID: 21490911
- PMCID: PMC3065733
- DOI: 10.1155/2010/978710
The antioxidant effect of erythropoietin on thalassemic blood cells
Abstract
Because of its stimulating effect on RBC production, erythropoietin (Epo) is used to treat anemia, for example, in patients on dialysis or on chemotherapy. In β-thalassemia, where Epo levels are low relative to the degree of anemia, Epo treatment improves the anemia state. Since RBC and platelets of these patients are under oxidative stress, which may be involved in anemia and thromboembolic complications, we investigated Epo as an antioxidant. Using flow-cytometry technology, we found that in vitro treatment with Epo of blood cells from these patients increased their glutathione content and reduced their reactive oxygen species, membrane lipid peroxides, and external phosphatidylserine. This resulted in reduced susceptibility of RBC to undergo hemolysis and phagocytosis. Injection of Epo into heterozygous (Hbb(th3/+)) β-thalassemic mice reduced the oxidative markers within 3 hours. Our results suggest that, in addition to stimulating RBC and fetal hemoglobin production, Epo might alleviate symptoms of hemolytic anemias as an antioxidant.
Figures
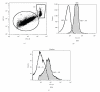
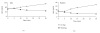


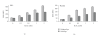


Similar articles
-
Fermented papaya preparation as redox regulator in blood cells of beta-thalassemic mice and patients.Phytother Res. 2008 Jun;22(6):820-8. doi: 10.1002/ptr.2379. Phytother Res. 2008. PMID: 18384199
-
Oxidative status of platelets in normal and thalassemic blood.Thromb Haemost. 2004 Nov;92(5):1052-9. doi: 10.1160/TH04-04-0234. Thromb Haemost. 2004. PMID: 15543333
-
Effects of a vitamin E-bonded membrane and of glutathione on anemia and erythropoietin requirements in hemodialysis patients.J Nephrol. 2002 Sep-Oct;15(5):558-64. J Nephrol. 2002. PMID: 12455724 Clinical Trial.
-
[Erythropoietin determination in clinical medicine].Rinsho Byori. 1993 Apr;41(4):361-5. Rinsho Byori. 1993. PMID: 8350498 Review. Japanese.
-
[Erythropoietin-beta in the treatment of anemia in patients with chronic renal insufficiency].Med Pregl. 2001 May-Jun;54(5-6):235-40. Med Pregl. 2001. PMID: 11759218 Review. Croatian.
Cited by
-
Oxidative DNA Damage, Inflammatory Signature, and Altered Erythrocytes Properties in Diamond-Blackfan Anemia.Int J Mol Sci. 2020 Dec 17;21(24):9652. doi: 10.3390/ijms21249652. Int J Mol Sci. 2020. PMID: 33348919 Free PMC article.
-
Erythropoietin Enhanced Recovery After Traumatic Nerve Injury: Myelination and Localized Effects.J Hand Surg Am. 2016 Oct;41(10):999-1010. doi: 10.1016/j.jhsa.2016.08.002. Epub 2016 Sep 1. J Hand Surg Am. 2016. PMID: 27593486 Free PMC article.
-
Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system.Int J Biol Sci. 2014 Aug 23;10(8):921-39. doi: 10.7150/ijbs.9518. eCollection 2014. Int J Biol Sci. 2014. PMID: 25170305 Free PMC article. Review.
-
AMPK is involved in mediation of erythropoietin influence on metabolic activity and reactive oxygen species production in white adipocytes.Int J Biochem Cell Biol. 2014 Sep;54:1-9. doi: 10.1016/j.biocel.2014.06.008. Epub 2014 Jun 19. Int J Biochem Cell Biol. 2014. PMID: 24953559 Free PMC article.
-
Deferoxamine Preconditioning of Neural-Like Cells Derived from Human Wharton's Jelly Mesenchymal Stem Cells as a Strategy to Promote Their Tolerance and Therapeutic Potential: An In Vitro Study.Cell Mol Neurobiol. 2016 Jul;36(5):689-700. doi: 10.1007/s10571-015-0249-8. Epub 2015 Aug 5. Cell Mol Neurobiol. 2016. PMID: 26242172 Free PMC article.
References
-
- Krantz SB. Erythropoietin. Blood. 1991;77(3):419–434. - PubMed
-
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiological Reviews. 1992;72(2):449–489. - PubMed
-
- Lodish HF, Hilton DJ, Klingmuller U, Watowich SS, Wu H. The erythropoietin receptor: biogenesis, dimerization, and intracellular signal transduction. Cold Spring Harbor Symposia on Quantitative Biology. 1995;60:93–104. - PubMed
-
- Beutel G, Ganser A. Risks and benefits of erythropoiesis-stimulating agents in cancer management. Seminars in Hematology. 2007;44(3):157–165. - PubMed
-
- Coladonato JA, Frankenfield DL, Reddan DN, et al. Trends in anemia management among US hemodialysis patients. Journal of the American Society of Nephrology. 2002;13(5):1288–1295. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

