Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing
- PMID: 21490918
- PMCID: PMC3072397
- DOI: 10.1371/journal.pone.0018410
Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing
Abstract
Background: Matricellular proteins, including periostin, are important for tissue regeneration.
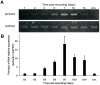
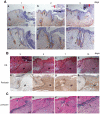
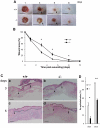
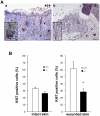
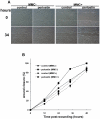
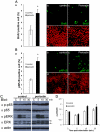
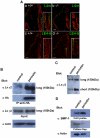
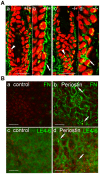
Methods and findings: Presently we investigated the function of periostin in cutaneous wound healing by using periostin-deficient ⁻/⁻ mice. Periostin mRNA was expressed in both the epidermis and hair follicles, and periostin protein was located at the basement membrane in the hair follicles together with fibronectin and laminin γ2. Periostin was associated with laminin γ2, and this association enhanced the proteolytic cleavage of the laminin γ2 long form to produce its short form. To address the role of periostin in wound healing, we employed a wound healing model using WT and periostin⁻/⁻ mice and the scratch wound assay in vitro. We found that the wound closure was delayed in the periostin⁻/⁻ mice coupled with a delay in re-epithelialization and with reduced proliferation of keratinocytes. Furthermore, keratinocyte proliferation was enhanced in periostin-overexpressing HaCaT cells along with up-regulation of phosphorylated NF-κB.
Conclusion: These results indicate that periostin was essential for keratinocyte proliferation for re-epithelialization during cutaneous wound healing.
Conflict of interest statement
Figures

References
-
- Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–87. - PubMed
-
- Eyden B. The myofibroblast, electron microscopy and cancer research. Int J Cancer. 2009;125:1743–1745. - PubMed
-
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 2005;11:1351–1354. - PubMed
-
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

