Some secrets of fluorescent proteins: distinct bleaching in various mounting fluids and photoactivation of cyan fluorescent proteins at YFP-excitation
- PMID: 21490932
- PMCID: PMC3072413
- DOI: 10.1371/journal.pone.0018586
Some secrets of fluorescent proteins: distinct bleaching in various mounting fluids and photoactivation of cyan fluorescent proteins at YFP-excitation
Abstract
Background: The use of spectrally distinct variants of green fluorescent protein (GFP) such as cyan or yellow mutants (CFP and YFP, respectively) is very common in all different fields of life sciences, e.g. for marking specific proteins or cells or to determine protein interactions. In the latter case, the quantum physical phenomenon of fluorescence resonance energy transfer (FRET) is exploited by specific microscopy techniques to visualize proximity of proteins.
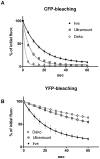
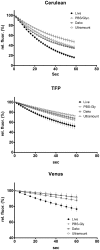
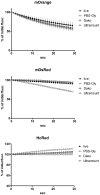
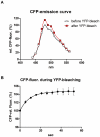
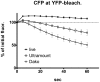
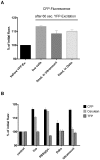
Methodology/principal findings: When we applied a commonly used FRET microscopy technique--the increase in donor (CFP)-fluorescence after bleaching of acceptor fluorophores (YFP), we obtained good signals in live cells, but very weak signals for the same samples after fixation and mounting in commercial microscopy mounting fluids. This observation could be traced back to much faster bleaching of CFP in these mounting media. Strikingly, the opposite effect of the mounting fluid was observed for YFP and also for other proteins such as Cerulean, TFP or Venus. The changes in photostability of CFP and YFP were not caused by the fixation but directly dependent on the mounting fluid. Furthermore we made the interesting observation that the CFP-fluorescence intensity increases by about 10-15% after illumination at the YFP-excitation wavelength--a phenomenon, which was also observed for Cerulean. This photoactivation of cyan fluorescent proteins at the YFP-excitation can cause false-positive signals in the FRET-microscopy technique that is based on bleaching of a yellow FRET acceptor.
Conclusions/significance: Our results show that photostability of fluorescent proteins differs significantly for various media and that CFP bleaches significantly faster in commercial mounting fluids, while the opposite is observed for YFP and some other proteins. Moreover, we show that the FRET microscopy technique that is based on bleaching of the YFP is prone to artifacts due to photoactivation of cyan fluorescent proteins under these conditions.
Conflict of interest statement
Figures


References
-
- Tsien RY. THE GREEN FLUORESCENT PROTEIN. Annual Review of Biochemistry. 1998;67:509–544. - PubMed
-
- Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol. 2003;21:1387–1395. - PubMed
-
- Schmid JA, Sitte HH. Fluorescence resonance energy transfer in the study of cancer pathways. CurrOpinOncol. 2003;15:55–64. - PubMed
-
- Van Munster EB, Kremers GJ, Adjobo-Hermans MJ, Gadella TW., Jr Fluorescence resonance energy transfer (FRET) measurement by gradual acceptor photobleaching. JMicrosc. 2005;218:253–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

