Down-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD
- PMID: 21490961
- PMCID: PMC3072378
- DOI: 10.1371/journal.pone.0014793
Down-regulation of the canonical Wnt β-catenin pathway in the airway epithelium of healthy smokers and smokers with COPD
Abstract
Background: The Wnt pathway mediates differentiation of epithelial tissues; depending on the tissue types, Wnt can either drive or inhibit the differentiation process. We hypothesized that key genes in the Wnt pathway are suppressed in the human airway epithelium under the stress of cigarette smoking, a stress associated with dysregulation of the epithelial differentiated state.
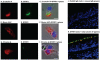
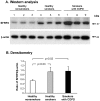
Methodology/principal findings: Microarrays were used to assess the expression of Wnt-related genes in the small airway epithelium (SAE) obtained via bronchoscopy and brushing of healthy nonsmokers, healthy smokers, and smokers with COPD. Thirty-three of 56 known Wnt-related genes were expressed in the SAE. Wnt pathway downstream mediators β-catenin and the transcription factor 7-like 1 were down-regulated in healthy smokers and smokers with COPD, as were many Wnt target genes. Among the extracellular regulators that suppress the Wnt pathway, secreted frizzled-related protein 2 (SFRP2), was up-regulated 4.3-fold in healthy smokers and 4.9-fold in COPD smokers, an observation confirmed by TaqMan Real-time PCR, Western analysis and immunohistochemistry. Finally, cigarette smoke extract mediated up-regulation of SFRP2 and down-regulation of Wnt target genes in airway epithelial cells in vitro.
Conclusions/significance: Smoking down-regulates the Wnt pathway in the human airway epithelium. In the context that Wnt pathway plays an important role in differentiation of epithelial tissues, the down-regulation of Wnt pathway may contribute to the dysregulation of airway epithelium differentiation observed in smoking-related airway disorders.
Conflict of interest statement
Figures

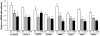
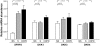


References
-
- Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

