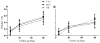A randomized trial of two behavioral interventions to improve outcomes following inpatient detoxification for alcohol dependence
- PMID: 21491295
- PMCID: PMC3126629
- DOI: 10.1080/10550887.2011.554777
A randomized trial of two behavioral interventions to improve outcomes following inpatient detoxification for alcohol dependence
Abstract
Participants (n=150), undergoing inpatient alcohol detoxification, were randomized into 3 groups: treatment as usual (TAU), motivation enhancement therapy (MET), or peer-delivered 12-step facilitation (P-TSF). The main outcome was the initiation of any type of subsequent rehabilitation service (i.e., professional treatment or self-help) within 30 and 90 days of discharge. At the 30-day follow-up interview, there was no significant difference among the groups in the rate of initiation of any type of subsequent care (82%, 74%, and 82%, respectively, p=0.617); however, the MET group had significantly more patients initiate subsequent inpatient treatment by the 90-day follow-up interview compared to the P-TSF group (31% and 61%, respectively, p=0.007) and a greater proportion of MET participants completed subsequent inpatient treatment compared to both the TAU and P-TSF groups. There were no differences in drinking-related outcomes. MET during inpatient detoxification may help patients initiate subsequent inpatient rehabilitation treatment.
Trial registration: ClinicalTrials.gov NCT00513708.
© Taylor & Francis Group, LLC
Figures
References
-
- Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348(18):1786–1795. - PubMed
-
- Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence; the COMBINE study; a randomized controlled trial. JAMA. 2006;295:2003–2017. - PubMed
-
- Foster JH, Marshall EJ, Peters TJ. Outcome after in-patient detoxification for alcohol dependence: A naturalistic comparison of 7 versus 28 days stay. Alcohol Alcohol. 2000;35(6):580–586. - PubMed
-
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144–151. - PubMed
-
- Millery M, Kleinman BP, Polissar NL, Millman RB, Scimeca M. Detoxification as a gateway to long-term treatment: assessing two interventions. J Subst Abuse Treat. 2002;23(3):183–190. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous