Medical interventions for high grade vulval intraepithelial neoplasia
- PMID: 21491403
- PMCID: PMC4170998
- DOI: 10.1002/14651858.CD007924.pub2
Medical interventions for high grade vulval intraepithelial neoplasia
Update in
-
Medical interventions for high-grade vulval intraepithelial neoplasia.Cochrane Database Syst Rev. 2015 Aug 18;2015(8):CD007924. doi: 10.1002/14651858.CD007924.pub3. Cochrane Database Syst Rev. 2015. PMID: 26284429 Free PMC article.
Abstract
Background: Vulval intraepithelial neoplasia (VIN) is a pre-malignant condition of the vulval skin; its incidence is increasing in women under 50 years. VIN is graded histologically as low grade or high grade. High grade VIN is associated with infection with human papilloma virus (HPV) infection and may progress to invasive disease. There is no consensus on the optimal management of high grade VIN. The high morbidity and high relapse rate associated with surgical interventions call for a formal appraisal of the evidence available for less invasive but effective interventions for high grade VIN.
Objectives: To evaluate the effectiveness and safety of medical interventions for high grade VIN.
Search strategy: We searched the Cochrane Gynaecological Cancer Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3), MEDLINE and EMBASE (up to September 2010). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria: Randomised controlled trials (RCTs) that assessed medical interventions, in adult women diagnosed with high grade VIN.
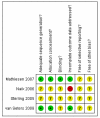
Data collection and analysis: Two review authors independently abstracted data and assessed risk of bias. Where possible the data were synthesised in a meta-analysis.
Main results: Four trials met our inclusion criteria: three assessed the effectiveness of topical imiquimod versus placebo in women with high grade VIN; one examined low versus high dose indole-3-carbinol in similar women. Meta-analysis of three trials found that the proportion of women who responded to treatment at 5 to 6 months was much higher in the group who received topical imiquimod than in the group who received placebo (relative risk (RR) = 11.95, 95% confidence interval (CI) 3.21 to 44.51). A single trial showed similar results at 12 months in (RR = 9.10, 95% CI 2.38 to 34.77). Only one trial reported adverse events, which were more common in the imiquimod group. One trial found no significant differences in quality of life (QoL) or body image between the imiquimod and placebo groups.
Authors' conclusions: Imiquimod appears to be effective, but its safety needs further examination. Its use is associated with side effects which are tolerable, but more extensive data on adverse effects are required. Long term follow-up should be mandatory in view of the known progression of high grade VIN to invasive disease. Alternative medical interventions, such as cidofovir, should be explored.
Figures
References
References to studies included in this review
-
- Mathiesen O, Buus SK, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: A randomised, double-blinded study. Gynecologic Oncology. 2007;107(2):219–22. - PubMed
-
- Naik R, Nixon S, Lopes A, Godfrey K, Hatem MH, Monaghan JM. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. International Journal of Gynecological Cancer. 2006;Vol. 16(issue 2):786–90. - PubMed
-
- Sterling JC, Smith NA, Loo WJ, Cohen C, Neill S, Nicholson A, et al. Randomized, double blind, placebo-controlled trial for treatment of high grade vulval intraepithelial neoplasia with imiquimod. Abstract FC06.1. Journal of the European Academy of Dermatology and Venereology. 2005;Vol. 19(issue Suppl 2):22.
-
- Terlou A, van Seters M, Ewing PC, Aaronson NK, Gundy CM, Heijmans-Antonissen C, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: Seven years median follow-up of a randomized clinical trial. Gynecologic Oncology. 2011 doi:10.1016/ j.ygyno.2010.12.340. - PubMed
- van Seters M, van Beurden M, Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. New England Journal of Medicine. 2008;358(14):1465–73. - PubMed
References to studies excluded from this review
-
- Iavazzo C, Pitsouni E, Athanasiou S, Falagas ME. Imiquimod for treatment of vulvar and vaginal intraepithelial neoplasia. International Journal of Gynaecology and Obstetrics. 2008;101(1):3–10. - PubMed
-
- Mahto M, Nathan M, O’Mahony C. More than a decade on: review of the use of imiquimod in lower anogenital intraepithelial neoplasia. International Journal of STD & AIDS. 2010;21(1):8–16. - PubMed
-
- Mathiesen O. Topical imiquimod can reverse vulvar intraepithelial neoplasia: A randomised, double blinded study - an answer. Gynecological Oncology. 2008;109(3):431. - PubMed
-
- Melamed EL. Precancerous lesions of the vulva and therapeutic methods. Data from the Leningrad Municipal oncological dispensary. [Russian] Voprosy. 1965:85–9. Onkologii. - PubMed
-
- Spirtos NM, Smith LH, Teng NN. Prospective randomized trial of topical alpha-interferon (alpha-interferon gels) for the treatment of vulvar intraepithelial neoplasia III. Gynecol Oncol. 1990;37(1):34–8. - PubMed
- Spirtos NM, Smith LH, Teng NNH. A prospective, randomized trial of topical interferon-alpha (INF) gels for the treatment of vulvar intraepithelial neoplasia III (VIN III) Gynecological Oncology. 1989;Vol. 32(issue 1):112. Abstract 76. - PubMed
References to ongoing studies
-
- Fiander A. A randomised phase II multi-centre clinical trial of topical treatment in women with vulval intraepithelial neoplasia. [RT3VIN] ISRCTN34420460
Additional references
-
- Andreasson B, Moth I, Jensen SB, Bock JE. Sexual function and somatopsychic reactions in vulvectomy-operated women and their partners. Acta Obstetricia et Gynecologica Scandinavica. 1986;65(1):7–10. - PubMed
-
- Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, et al. Placebo-Controlled Trial of Indole-3-Carbinol in the Treatment of CIN. Gynecologic Oncology. 2000;78(2):123–9. - PubMed
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of Clinical Epidemiology. 1997;50(6):683–91. - PubMed
-
- Higgins JPT, Green S, editors. The Cochrane Collaboration. issue Version 5.0.1. 2008. Cochrane Handbook for Systematic Reviews of Interventions.www.cochrane–handbook.org
-
- CTCAE . Common Terminology Criteria for Adverse Events. Vol. v3.0. CTCAE; Aug 9th, 2006. http://ctep.cancer.gov/forms/CTCAEv3.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous