The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies
- PMID: 21491437
- PMCID: PMC3164548
- DOI: 10.1002/jps.22432
The utility of hydrogen/deuterium exchange mass spectrometry in biopharmaceutical comparability studies
Abstract
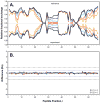
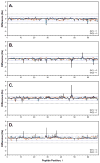
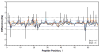
The function, efficacy, and safety of protein biopharmaceuticals are tied to their three-dimensional structure. The analysis and verification of this higher-order structure are critical in demonstrating manufacturing consistency and in establishing the absence of structural changes in response to changes in production. It is, therefore, essential to have reliable, high-resolution and high sensitivity biophysical tools capable of interrogating protein structure and conformation. Here, we demonstrate the use of hydrogen/deuterium exchange mass spectrometry (H/DX-MS) in biopharmaceutical comparability studies. H/DX-MS measurements can be conducted with good precision, consume only picomoles of protein, interrogate nearly the entire molecule with peptide level resolution, and can be completed in a few days. Structural comparability or lack of comparability was monitored for different preparations of interferon-β-1a. We present specific graphical formats for the display of H/DX-MS data that aid in rapidly making both the qualitative (visual) and quantitative assessment of comparability. H/DX-MS is capable of making significant contributions in biopharmaceutical characterization by providing more informative and confidant comparability assessments of protein higher-order structures than are currently available within the biopharmaceutical industry.
Copyright © 2010 Wiley-Liss, Inc.
Figures
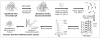


References
-
- Lundblad RL. Approaches to the Conformational Analysis of Biopharmaceuticals. Boca Raton, FL: Chapman & Hall/CRC Press; 2009. Dec 15, p. 336.
-
- Rader RA. (Re)defining biopharmaceutical. Nat Biotechnol. 2008;26(7):743–751. - PubMed
-
- Zuñiga L, Calvo B. Biosimilars approval process. Regul Toxicol Pharmacol. 2010;56(3):374–377. - PubMed
-
- Schellekens H, Moors E. Clinical comparability and European biosimilar regulations. Nat Biotechnol. 2010;28(1):28–31. - PubMed
-
- ICH Q5E guidelines. http://www.ich.org/LOB/media/MEDIA1196.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

