Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina
- PMID: 21491544
- PMCID: PMC4263955
- DOI: 10.1002/stem.637
Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina
Abstract
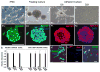
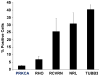
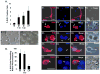
Absence of a regenerative pathway for damaged retina following injury or disease has led to experiments using stem cell transplantation for retinal repair, and encouraging results have been obtained in rodents. The swine eye is a closer anatomical and physiological match to the human eye, but embryonic stem cells have not been isolated from pig, and photoreceptor differentiation has not been demonstrated with induced pluripotent stem cells (iPSCs) of swine. Here, we subjected iPSCs of swine to a rod photoreceptor differentiation protocol consisting of floating culture as embryoid bodies followed by differentiation in adherent culture. Real-time PCR and immunostaining of differentiated cells demonstrated loss of expression of the pluripotent genes POU5F1, NANOG, and SOX2 and induction of rod photoreceptor genes RCVRN, NRL, RHO, and ROM1. While these differentiated cells displayed neuronal morphology, culturing on a Matrigel substratum triggered a further morphological change resulting in concentration of rhodopsin (RHO) and rod outer segment-specific membrane protein 1 in outer segment-like projections resembling those on primary cultures of rod photoreceptors. The differentiated cells were transplanted into the subretinal space of pigs treated with iodoacetic acid to eliminate rod photoreceptors. Three weeks after transplantation, engrafted RHO+ cells were evident in the outer nuclear layer where photoreceptors normally reside. A portion of these transplanted cells had generated projections resembling outer segments. These results demonstrate that iPSCs of swine can differentiate into photoreceptors in culture, and these cells can integrate into the damaged swine neural retina, thus, laying a foundation for future studies using the pig as a model for retinal stem cell transplantation.
Copyright © 2011 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures



Similar articles
-
Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors.Stem Cells. 2013 Jun;31(6):1149-59. doi: 10.1002/stem.1372. Stem Cells. 2013. PMID: 23495178 Free PMC article.
-
Swine cone and rod precursors arise sequentially and display sequential and transient integration and differentiation potential following transplantation.Invest Ophthalmol Vis Sci. 2014 Jan 15;55(1):301-9. doi: 10.1167/iovs.13-12600. Invest Ophthalmol Vis Sci. 2014. PMID: 24327609 Free PMC article.
-
Transcriptome Dynamics of Developing Photoreceptors in Three-Dimensional Retina Cultures Recapitulates Temporal Sequence of Human Cone and Rod Differentiation Revealing Cell Surface Markers and Gene Networks.Stem Cells. 2015 Dec;33(12):3504-18. doi: 10.1002/stem.2122. Epub 2015 Aug 14. Stem Cells. 2015. PMID: 26235913 Free PMC article.
-
Using induced pluripotent stem cells to understand retinal ciliopathy disease mechanisms and develop therapies.Biochem Soc Trans. 2016 Oct 15;44(5):1245-1251. doi: 10.1042/BST20160156. Biochem Soc Trans. 2016. PMID: 27911706 Free PMC article. Review.
-
Deciphering retinal diseases through the generation of three dimensional stem cell-derived organoids: Concise Review.Stem Cells. 2019 Dec;37(12):1496-1504. doi: 10.1002/stem.3089. Epub 2019 Oct 31. Stem Cells. 2019. PMID: 31617949 Free PMC article. Review.
Cited by
-
Induced pluripotent stem cells as custom therapeutics for retinal repair: progress and rationale.Exp Eye Res. 2014 Jun;123:161-72. doi: 10.1016/j.exer.2013.12.001. Epub 2014 Feb 16. Exp Eye Res. 2014. PMID: 24534198 Free PMC article. Review.
-
Intravitreal Injection of Human Retinal Progenitor Cells for Treatment of Retinal Degeneration.Med Sci Monit. 2020 Mar 28;26:e921184. doi: 10.12659/MSM.921184. Med Sci Monit. 2020. PMID: 32221273 Free PMC article.
-
Using human induced pluripotent stem cells to treat retinal disease.Prog Retin Eye Res. 2013 Nov;37:163-81. doi: 10.1016/j.preteyeres.2013.09.002. Epub 2013 Oct 6. Prog Retin Eye Res. 2013. PMID: 24104210 Free PMC article. Review.
-
Two-Step Reactivation of Dormant Cones in Retinitis Pigmentosa.Cell Rep. 2016 Apr 12;15(2):372-85. doi: 10.1016/j.celrep.2016.03.022. Epub 2016 Mar 31. Cell Rep. 2016. PMID: 27050517 Free PMC article.
-
Modeling Keratoconus Using Induced Pluripotent Stem Cells.Invest Ophthalmol Vis Sci. 2016 Jul 1;57(8):3685-97. doi: 10.1167/iovs.16-19105. Invest Ophthalmol Vis Sci. 2016. PMID: 27403997 Free PMC article.
References
-
- Lamba DA, Karl MO, Reh TA. Strategies for retinal repair: cell replacement and regeneration. Prog Brain Res. 2009;175:23–31. - PubMed
-
- Jin ZB, Okamoto S, Mandai M, et al. Induced pluripotent stem cells for retinal degenerative diseases: a new perspective on the challenges. J Genet. 2009;88:417–423. - PubMed
-
- Bull ND, Martin KR. Using stem cells to mend the retina in ocular disease. Regen Med. 2009;4:855–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

