Glyoxalase I polymorphism rs2736654 causing the Ala111Glu substitution modulates enzyme activity--implications for autism
- PMID: 21491613
- PMCID: PMC3138858
- DOI: 10.1002/aur.197
Glyoxalase I polymorphism rs2736654 causing the Ala111Glu substitution modulates enzyme activity--implications for autism
Abstract
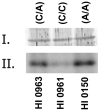
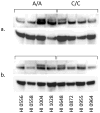
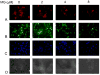
Autism is a pervasive, heterogeneous, neurodevelopmental disability characterized by impairments in verbal communications, reciprocal social interactions, and restricted repetitive stereotyped behaviors. Evidence suggests the involvement of multiple genetic factors in the etiology of autism, and extensive genome-wide association studies have revealed several candidate genes that bear single nucleotide polymorphisms (SNPs) in non-coding and coding regions. We have shown that a non-conservative, non-synonymous SNP in the glyoxalase I gene, GLOI, may be an autism susceptibility factor. The GLOI rs2736654 SNP is a C→A change that causes an Ala111Glu change in the Glo1 enzyme. To identify the significance of the SNP, we have conducted functional assays for Glo1. We now present evidence that the presence of the A-allele at rs2736654 results in reduced enzyme activity. Glo1 activity is decreased in lymphoblastoid cells that are homozygous for the A allele. The Glu-isoform of Glo1 in these cells is hyperphosphorylated. Direct HPLC measurements of the glyoxalase I substrate, methylglyoxal (MG), show an increase in MG in these cells. Western blot analysis revealed elevated levels of the receptor for advanced glycation end products (RAGEs). We also show that MG is toxic to the developing neuronal cells. We suggest that accumulation of MG results in the formation of AGEs, which induce expression of the RAGE that during crucial neuronal development may be a factor in the pathology of autism.
Copyright © 2011, International Society for Autism Research, Wiley-Liss, Inc.
Figures
Similar articles
-
Proteomic studies identified a single nucleotide polymorphism in glyoxalase I as autism susceptibility factor.Am J Med Genet A. 2004 Nov 15;131(1):11-7. doi: 10.1002/ajmg.a.30349. Am J Med Genet A. 2004. PMID: 15386471 Free PMC article.
-
The GLO1 C332 (Ala111) allele confers autism vulnerability: family-based genetic association and functional correlates.J Psychiatr Res. 2014 Dec;59:108-16. doi: 10.1016/j.jpsychires.2014.07.021. Epub 2014 Aug 8. J Psychiatr Res. 2014. PMID: 25201284
-
Case-control and family-based association studies of candidate genes in autistic disorder and its endophenotypes: TPH2 and GLO1.BMC Med Genet. 2007 Mar 8;8:11. doi: 10.1186/1471-2350-8-11. BMC Med Genet. 2007. PMID: 17346350 Free PMC article.
-
The Role of Glyoxalase in Glycation and Carbonyl Stress Induced Metabolic Disorders.Curr Protein Pept Sci. 2020;21(9):846-859. doi: 10.2174/1389203721666200505101734. Curr Protein Pept Sci. 2020. PMID: 32368974 Review.
-
Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators.Biomed Pharmacother. 2020 Nov;131:110663. doi: 10.1016/j.biopha.2020.110663. Epub 2020 Aug 25. Biomed Pharmacother. 2020. PMID: 32858501
Cited by
-
Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions.Sci Rep. 2017 Jul 11;7(1):5126. doi: 10.1038/s41598-017-05287-z. Sci Rep. 2017. PMID: 28698611 Free PMC article.
-
Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway.Redox Biol. 2018 Apr;14:465-473. doi: 10.1016/j.redox.2017.10.015. Epub 2017 Oct 18. Redox Biol. 2018. PMID: 29080525 Free PMC article. Review.
-
Transcriptome study of differential expression in schizophrenia.Hum Mol Genet. 2013 Dec 15;22(24):5001-14. doi: 10.1093/hmg/ddt350. Epub 2013 Jul 30. Hum Mol Genet. 2013. PMID: 23904455 Free PMC article.
-
Polymorphisms in Glyoxalase I Gene Are Not Associated with Glyoxalase I Expression in Whole Blood or Markers of Methylglyoxal Stress: The CODAM Study.Antioxidants (Basel). 2021 Feb 2;10(2):219. doi: 10.3390/antiox10020219. Antioxidants (Basel). 2021. PMID: 33540757 Free PMC article.
-
Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal.J Clin Invest. 2012 Jun;122(6):2306-15. doi: 10.1172/JCI61319. Epub 2012 May 15. J Clin Invest. 2012. PMID: 22585572 Free PMC article.
References
-
- Ahmed N, Battah S, Karachalias N, Babaei-Jadidi R, Horanyi M, Baroti K, et al. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochimica et Biophysica Acta. 2003;1639:121–132. - PubMed
-
- Amicarelli F, Sacchetta P, Colafarina S, Angelucci S, Miranda M, DeIlio C. Glyoxalases activity during Bufo bufo embryo development. Mechanisms of Ageing and Development. 1998;100:261–267. - PubMed
-
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. International Journal of Developmental Neuroscience. 2005;23:183–187. - PubMed
-
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. Journal of Molecular Biology. 1999;294:1351–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

