Glyoxalase I polymorphism rs2736654 causing the Ala111Glu substitution modulates enzyme activity--implications for autism
- PMID: 21491613
- PMCID: PMC3138858
- DOI: 10.1002/aur.197
Glyoxalase I polymorphism rs2736654 causing the Ala111Glu substitution modulates enzyme activity--implications for autism
Abstract
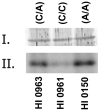
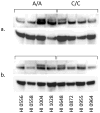
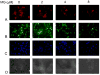
Autism is a pervasive, heterogeneous, neurodevelopmental disability characterized by impairments in verbal communications, reciprocal social interactions, and restricted repetitive stereotyped behaviors. Evidence suggests the involvement of multiple genetic factors in the etiology of autism, and extensive genome-wide association studies have revealed several candidate genes that bear single nucleotide polymorphisms (SNPs) in non-coding and coding regions. We have shown that a non-conservative, non-synonymous SNP in the glyoxalase I gene, GLOI, may be an autism susceptibility factor. The GLOI rs2736654 SNP is a C→A change that causes an Ala111Glu change in the Glo1 enzyme. To identify the significance of the SNP, we have conducted functional assays for Glo1. We now present evidence that the presence of the A-allele at rs2736654 results in reduced enzyme activity. Glo1 activity is decreased in lymphoblastoid cells that are homozygous for the A allele. The Glu-isoform of Glo1 in these cells is hyperphosphorylated. Direct HPLC measurements of the glyoxalase I substrate, methylglyoxal (MG), show an increase in MG in these cells. Western blot analysis revealed elevated levels of the receptor for advanced glycation end products (RAGEs). We also show that MG is toxic to the developing neuronal cells. We suggest that accumulation of MG results in the formation of AGEs, which induce expression of the RAGE that during crucial neuronal development may be a factor in the pathology of autism.
Copyright © 2011, International Society for Autism Research, Wiley-Liss, Inc.
Figures
References
-
- Ahmed N, Battah S, Karachalias N, Babaei-Jadidi R, Horanyi M, Baroti K, et al. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochimica et Biophysica Acta. 2003;1639:121–132. - PubMed
-
- Amicarelli F, Sacchetta P, Colafarina S, Angelucci S, Miranda M, DeIlio C. Glyoxalases activity during Bufo bufo embryo development. Mechanisms of Ageing and Development. 1998;100:261–267. - PubMed
-
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. International Journal of Developmental Neuroscience. 2005;23:183–187. - PubMed
-
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. Journal of Molecular Biology. 1999;294:1351–1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

