Substantial contribution of the two imidazole rings of the His13-His14 dyad to Cu(II) binding in amyloid-β(1-16) at physiological pH and its significance
- PMID: 21491887
- PMCID: PMC3155642
- DOI: 10.1021/jp200379m
Substantial contribution of the two imidazole rings of the His13-His14 dyad to Cu(II) binding in amyloid-β(1-16) at physiological pH and its significance
Abstract
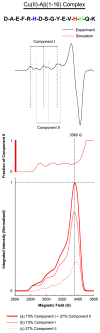
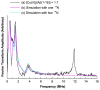
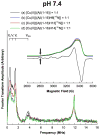
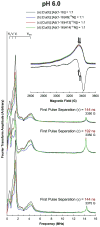
The interaction of amyloid-β (Aβ) peptide with Cu(II) appears to play an important role in the etiology of Alzheimer's disease. At physiological pH, the Cu(II) coordination in Aβ is heterogeneous, and there exist at least two binding modes in which Cu(II) is coordinated by histidine residues. Electron spin resonance studies have revealed a picture of the Cu(II) binding at a higher or lower pH, where only one of the two binding modes is almost exclusively present. We describe a procedure to directly examine the coordination of Cu(II) to each histidine residue in the dominant binding mode at physiological pH. We use nonlabeled and residue-specifically (15)N-labeled Aβ(1-16). For quantitative analysis, the intensities of three-pulse electron spin-echo envelope modulation (ESEEM) spectra are analyzed. Spectral simulations show that ESEEM intensities provide information about the contribution of each histidine residue. Indeed, the ESEEM experiments at pH 6.0 confirm the dominant contribution of His6 to the Cu(II) coordination as expected from the work of other researchers. Interestingly, however, the ESEEM data obtained at pH 7.4 reveal that the contributions of the three residues to the Cu(II) coordination are in the order of His14 ≈ His6 > His13 in the dominant binding mode. The order indicates a significant contribution from the simultaneous coordination by His13 and His14 at physiological pH, which has been underappreciated. These findings are supported by hyperfine sublevel correlation spectroscopy experiments. The simultaneous coordination by the two adjacent residues is likely to be present in a non-β-sheet structure. The coexistence of different secondary structures is possibly the molecular origin for the formation of amorphous aggregates rather than fibrils at relatively high concentrations of Cu(II). Through our approach, precise and useful information about Cu(II) binding in Aβ(1-16) at physiological pH is obtained without any side-chain modification, amino acid residue replacement, or pH change, each of which might lead to an alteration in the peptide structure or the coordination environment.
© 2011 American Chemical Society
Figures
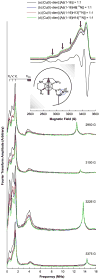

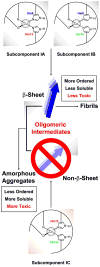
References
-
- Hardy J, Selkoe DJ. Science. 2002;297:353–356. - PubMed
-
- Kirkitadze MD, Bitan G, Teplow DB. J Neurosci Res. 2002;69:567–577. - PubMed
-
- Carrotta R, Manno M, Bulone D, Martorana V, Biagio PLS. J Biol Chem. 2005;280:30001–30008. - PubMed
-
- Haass C, Selkoe DJ. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Crouch PJ, Harding SME, White AR, Camakaris J, Bush AI, Masters CL. Int J Biochem Cell Biol. 2008;40:181–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

