Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy
- PMID: 21492240
- PMCID: PMC3164919
- DOI: 10.1111/j.1600-0846.2011.00508.x
Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy
Abstract
Background: In vivo confocal scanning laser microscopy (CSLM) is a recently developed non-invasive technique for visualizing microscopic structures with the skin. CSLM has been used to characterize proliferative and inflammatory skin diseases, neoplastic skin lesions and pigmented lesions.
Objective: Here, we assessed the ability of CSLM to evaluate the formation of neogenic hair follicles after a full-thickness wound in mice.
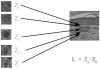
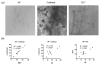
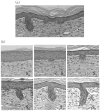
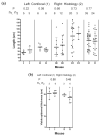
Methods: Full-thickness wounds were made on the dorsal skin of 3-week-old mice. After scab detachment (SD), the number, width, length, space and volume of neogenic hair follicles were analyzed using CSLM. The results were compared with those from conventional methods, including staining for alkaline phosphatase (AP) and keratin 17 (K17) as well as histology.
Results: Quantification of neogenic hair follicles using CSLM compared favorably with the results from direct measurements on isolated epidermal tissue after immunostaining for K17, a marker for the epithelial portion of new hair follicles. CSLM detected 89% of K17-stained follicles. CSLM more accurately quantified the number of new follicles compared with AP staining, which detects the dermal portion of the new follicle. The width and length measurement from CSLM and histology were very close and correlated with each other. The minimum length of a neogenic hair follicle that could be detected by CSLM was 21 μm. The space between neogenic hair follicles was decreased in histological sections compared with CSLM.
Conclusion: CSLM is an accurate and valuable method for counting and measuring neogenic hair follicles non-invasively. CSLM produces images similar to histology in mice. Measurements of microstructures using CSLM more accurately reflect actual sizes as this technique avoids fixation artifacts. In vivo visualization of developing follicles with CSLM allows the detection of serial changes in hair follicle formation, thus conserving the numbers of mice required for studies and improving the detection of temporal changes in developing hair follicles.
© 2011 John Wiley & Sons A/S.
Figures




References
-
- Billingham RE, Russell PS. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits' skin. Nature. 1956 Apr 28;177(4513):791–2. - PubMed
-
- Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954 Sep;14(8):575–9. - PubMed
-
- Lacassagne A, Latarjet R. Action of methylcholanthrene on certain scars of the skin in mice. Cancer Res. 1946;(6):183–8. - PubMed
-
- Kligman AM, Strauss JS. The formation of vellus hair follicles from human adult epidermis. J Invest Dermatol. 1956 Jul;27(1):19–23. - PubMed
-
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007 May 17;447(7142):316–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

