A trypsin-like serine protease is involved in pseudorabies virus invasion through the basement membrane barrier of porcine nasal respiratory mucosa
- PMID: 21492440
- PMCID: PMC3089791
- DOI: 10.1186/1297-9716-42-58
A trypsin-like serine protease is involved in pseudorabies virus invasion through the basement membrane barrier of porcine nasal respiratory mucosa
Abstract
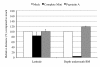
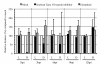
Several alphaherpesviruses breach the basement membrane during mucosal invasion. In the present study, the role of proteases in this process was examined. The serine protease-specific inhibitor AEBSF inhibited penetration of the basement membrane by the porcine alphaherpesvirus pseudorabies virus (PRV) by 88.1% without affecting lateral spread. Inhibitors of aspartic-, cysteine-, and metalloproteases did not inhibit viral penetration of the basement membrane. Further analysis using the Soybean Type I-S trypsin inhibitor for the serine protease subcategory of trypsin-like serine proteases resulted in a 96.9% reduction in plaque depth underneath the basement membrane. These data reveal a role of a trypsin-like serine protease in PRV penetration of the basement membrane.
Figures


Similar articles
-
Different replication characteristics of historical pseudorabies virus strains in porcine respiratory nasal mucosa explants.Vet Microbiol. 2009 May 12;136(3-4):341-6. doi: 10.1016/j.vetmic.2008.11.005. Epub 2008 Nov 18. Vet Microbiol. 2009. PMID: 19111405
-
The US3 Protein of Pseudorabies Virus Drives Viral Passage across the Basement Membrane in Porcine Respiratory Mucosa Explants.J Virol. 2016 Nov 14;90(23):10945-10950. doi: 10.1128/JVI.01577-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681139 Free PMC article.
-
The alphaherpesvirus gE/gI glycoprotein complex and proteases jointly orchestrate invasion across the host's upper respiratory epithelial barrier.mBio. 2024 Nov 13;15(11):e0187324. doi: 10.1128/mbio.01873-24. Epub 2024 Oct 9. mBio. 2024. PMID: 39382295 Free PMC article.
-
Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine.Microbiol Mol Biol Rev. 2005 Sep;69(3):462-500. doi: 10.1128/MMBR.69.3.462-500.2005. Microbiol Mol Biol Rev. 2005. PMID: 16148307 Free PMC article. Review.
-
Cell biological and molecular characteristics of pseudorabies virus infections in cell cultures and in pigs with emphasis on the respiratory tract.Vet Res. 2007 Mar-Apr;38(2):229-41. doi: 10.1051/vetres:200661. Epub 2007 Jan 25. Vet Res. 2007. PMID: 17257571 Review.
Cited by
-
CCL2 and CCL5 driven attraction of CD172a+ monocytic cells during an equine herpesvirus type 1 (EHV-1) infection in equine nasal mucosa and the impact of two migration inhibitors, rosiglitazone (RSG) and quinacrine (QC).Vet Res. 2017 Feb 27;48(1):14. doi: 10.1186/s13567-017-0419-4. Vet Res. 2017. PMID: 28241864 Free PMC article.
-
Replication characteristics of equine herpesvirus 1 and equine herpesvirus 3: comparative analysis using ex vivo tissue cultures.Vet Res. 2016 Jan 15;47:19. doi: 10.1186/s13567-016-0305-5. Vet Res. 2016. PMID: 26768993 Free PMC article.
-
Reduced virulence of a pseudorabies virus isolate from wild boar origin in domestic pigs correlates with hampered visceral spread and age-dependent reduced neuroinvasive capacity.Virulence. 2018 Jan 1;9(1):149-162. doi: 10.1080/21505594.2017.1368941. Epub 2017 Oct 4. Virulence. 2018. PMID: 28873002 Free PMC article.
-
Immobilization of pseudorabies virus in porcine tracheal respiratory mucus revealed by single particle tracking.PLoS One. 2012;7(12):e51054. doi: 10.1371/journal.pone.0051054. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236432 Free PMC article.
-
Diverse microbial interactions with the basement membrane barrier.Trends Microbiol. 2012 Mar;20(3):147-55. doi: 10.1016/j.tim.2012.01.001. Epub 2012 Jan 31. Trends Microbiol. 2012. PMID: 22300759 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

