Limited accessibility to designs and results of Japanese large-scale clinical trials for cardiovascular diseases
- PMID: 21492452
- PMCID: PMC3096578
- DOI: 10.1186/1745-6215-12-96
Limited accessibility to designs and results of Japanese large-scale clinical trials for cardiovascular diseases
Abstract
Background: Clinical evidence is important for improving the treatment of patients by health care providers. In the study of cardiovascular diseases, large-scale clinical trials involving thousands of participants are required to evaluate the risks of cardiac events and/or death. The problems encountered in conducting the Japanese Acute Myocardial Infarction Prospective (JAMP) study highlighted the difficulties involved in obtaining the financial and infrastructural resources necessary for conducting large-scale clinical trials. The objectives of the current study were: 1) to clarify the current funding and infrastructural environment surrounding large-scale clinical trials in cardiovascular and metabolic diseases in Japan, and 2) to find ways to improve the environment surrounding clinical trials in Japan more generally.
Methods: We examined clinical trials examining cardiovascular diseases that evaluated true endpoints and involved 300 or more participants using Pub-Med, Ichushi (by the Japan Medical Abstracts Society, a non-profit organization), websites of related medical societies, the University Hospital Medical Information Network (UMIN) Clinical Trials Registry, and clinicaltrials.gov at three points in time: 30 November, 2004, 25 February, 2007 and 25 July, 2009.
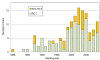
Results: We found a total of 152 trials that met our criteria for 'large-scale clinical trials' examining cardiovascular diseases in Japan. Of these, 72.4% were randomized controlled trials (RCTs). Of 152 trials, 9.2% of the trials examined more than 10,000 participants, and 42.8% examined between 1,000 and 10,000 participants. The number of large-scale clinical trials markedly increased from 2001 to 2004, but suddenly decreased in 2007, then began to increase again. Ischemic heart disease (39.5%) was the most common target disease. Most of the larger-scale trials were funded by private organizations such as pharmaceutical companies. The designs and results of 13 trials were not disclosed.
Conclusions: To improve the quality of clinical trials, all sponsors should register trials and disclose the funding sources before the enrolment of participants, and publish their results after the completion of each study.
Figures

References
-
- Anon. Evaluation of drug treatment in mild hypertension: VA-NHLBI feasibility trial. Plan and preliminary results of a two-year feasibility trial for a multicenter intervention study to evaluate the benefits versus the disadvantages of treating mild hypertension. Prepared for the Veterans Administration-National Heart, Lung, and Blood Institute Study Group for Evaluating Treatment in Mild Hypertension. Ann N Y Acad Sci. 1978;304:267–292. doi: 10.1111/j.1749-6632.1978.tb25604.x. - DOI - PubMed
-
- Ueshima K, Fukami K, Hiramori K, Hosoda S, Kishida H, Kato K, Fujita T, Tsutani K, Sakuma A. Japanese Acute Myocardial Infarction Prospective study group. Is angiotensin-converting enzyme inhibitor useful in a Japanese population for secondary prevention after acute myocardial infarction? A final report of the Japanese Acute Myocardial Infarction Prospective (JAMP) study. Am Heart J. 2004;148:292–299. doi: 10.1016/j.ahj.2004.03.035. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

