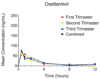
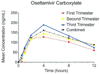
Pharmacokinetics of oseltamivir according to trimester of pregnancy
- PMID: 21492824
- PMCID: PMC3111855
- DOI: 10.1016/j.ajog.2011.03.005
Pharmacokinetics of oseltamivir according to trimester of pregnancy
Abstract
The purpose of this study was to determine pharmacokinetic parameters for oseltamivir in all trimesters of pregnancy. Thirty pregnant women, 10 per trimester, who were receiving oseltamivir phosphate (75 mg) were recruited to study first-dose pharmacokinetics. Plasma samples were obtained at 0, 0.5, 1, 2, 4, 8, and 12 hours after the first dose. Samples were analyzed for oseltamivir and oseltamivir carboxylate levels. With the use of a noncompartmental model, we estimated the area-under-the-curve, maximum concentration, time-to-maximum concentration, and half-life. There were no significant differences in the pharmacokinetics of oseltamivir by trimester, except for an increased half-life in the first trimester for oseltamivir phosphate and an increased maximum concentration in the third trimester for oseltamivir carboxylate. The levels of oseltamivir carboxylate that were observed were within the range that was needed to achieve inhibitory concentrations at 50% for pandemic H1N1. The pharmacokinetics of oseltamivir does not change significantly according to trimester of pregnancy.
Copyright © 2011 Mosby, Inc. All rights reserved.
Figures
Comment in
-
Oseltamivir pharmacokinetics in pregnancy: a commentary.Am J Obstet Gynecol. 2011 Jun;204(6 Suppl 1):S94-5. doi: 10.1016/j.ajog.2011.02.043. Epub 2011 Feb 23. Am J Obstet Gynecol. 2011. PMID: 21640232 No abstract available.
References
-
- Jamison DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 Influenza virus infection during pregnancy in the USA. The Lancet. 2009;374:451–458. - PubMed
-
- Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 Influenza A and Pregnancy Outcomes in Victoria, Australia. Clin Infect Dis. 2010;50:686–690. - PubMed
-
- Louie JK, Acosta M, Jamieson DJ, Honein M. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. New Eng J Med. 2010;362(1):27–35. - PubMed
-
- Archer BN, Cohen C, Naidoo D, et al. Interim Report on Pandemic H1N1 influenza virus infections in South Africa, April to October 2009: Epidemiology and Factors Associated with Fatal Cases. Euro Surveill. 2009;14(42) pii=19369. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical