A link between smooth muscle cell death and extracellular matrix degradation during vascular atrophy
- PMID: 21493032
- PMCID: PMC3129478
- DOI: 10.1016/j.jvs.2010.12.070
A link between smooth muscle cell death and extracellular matrix degradation during vascular atrophy
Abstract
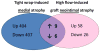
Objective: High blood flow induces neointimal atrophy in polytetrafluoroethylene (PTFE) aortoiliac grafts and a tight external PTFE wrap of the iliac artery induces medial atrophy. In both nonhuman primate models, atrophy with loss of smooth muscle cells and extracellular matrix (ECM) begins at ≤4 days. We hypothesized that matrix loss would be linked to cell death, but the factors and mechanisms involved are not known. The purpose of this study was to determine commonly regulated genes in these two models, which we hypothesized would be a small set of genes that might be key regulators of vascular atrophy.
Methods: DNA microarray analysis (Sentrix Human Ref 8; Illumina, San Diego, Calif; ∼23,000 genes) was performed on arterial tissue from the wrap model (n = 9) and graft neointima from the graft model (n = 5) 1 day after wrapping or the switch to high flow, respectively. Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was also performed. Expression of this vascular atrophy gene set was also studied after Fas ligand-induced cell death in cultured smooth muscle cells and organ cultured arteries.
Results: Microarray analysis showed 15 genes were regulated in the same direction in both atrophy models: 9 upregulated and 6 downregulated. Seven of nine upregulated genes were confirmed by qRT-PCR in both models. Upregulated genes included the ECM-degrading enzymes ADAMTS4, tissue plasminogen activator (PLAT), and hyaluronidase 2; possible growth regulatory factors, including chromosome 8 open reading frame 4 and leucine-rich repeat family containing 8; a differentiation regulatory factor (musculoskeletal embryonic nuclear protein 1); a dead cell removal factor (ficolin 3); and a prostaglandin transporter (solute carrier organic anion transporter family member 2A1). Five downregulated genes were confirmed but only in one or the other model. Of the seven upregulated genes, ADAMTS4, PLAT, hyaluronidase 2, solute carrier organic anion transporter family member 2A1, leucine-rich repeat family containing 8, and chromosome 8 open reading frame 4 were also upregulated in vitro in cultured smooth muscle cells or cultured iliac artery by treatment with FasL, which causes cell death. However, blockade of caspase activity with Z-VAD inhibited FasL-mediated cell death, but not gene induction.
Conclusion: Seven gene products were upregulated in two distinctly different in vivo nonhuman primate vascular atrophy models. Induction of cell death by FasL in vitro induced six of these genes, including the ECM-degrading factors ADAMTS4, hyaluronidase 2, and PLAT, suggesting a mechanism by which the program of tissue atrophy coordinately removes extracellular matrix as cells die. These genes may be key regulators of vascular atrophy.
Copyright © 2011 Society for Vascular Surgery. Published by Mosby, Inc. All rights reserved.
Figures




References
-
- Finn AV, Gold HK, Tang A, Weber DK, Wight TN, Clermont A, et al. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. Journal of Vascular Research. 2002;39(5):414–25. - PubMed
-
- Kim WH, Hong MK, Virmani R, Kornowski R, Jones R, Leon MB. Histopathologic analysis of in-stent neointimal regression in a porcine coronary model. CoronArtery Dis. 2000;11(3):273–7. - PubMed
-
- Kuroda N, Kobayashi Y, Nameki M, Kuriyama N, Kinoshita T, Okuno T, et al. Intimal hyperplasia regression from 6 to 12 months after stenting. Am J Cardiol. 2002;89(7):869–72. - PubMed
-
- Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, et al. Remodeling of in-stent neointima, which became thinner and transparent over 3 years - Serial angiographic and angioscopic follow-up. Circulation. 1998;97(20):2003–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

