Eribulin -- a review of preclinical and clinical studies
- PMID: 21493087
- PMCID: PMC3954568
- DOI: 10.1016/j.critrevonc.2011.03.002
Eribulin -- a review of preclinical and clinical studies
Abstract
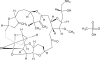
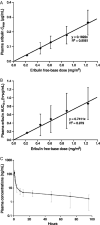
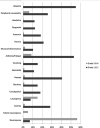
Eribulin mesylate is a non-taxane, structurally simplified, completely synthetic, halichondrin B derivative with an end poisoning, microtubule inhibitory action. Preclinical studies have demonstrated activity in various cancer cell lines and synergistic action with gemcitabine, epirubicin, trastuzumab, cisplatin, docetaxel and vinorelbine. Eribulin has recently been approved by United States Food and Drug Administration as a third line therapy for metastatic breast cancer patients, who have previously been treated with an anthracycline and a taxane. It has also advanced to phase II trials in non-small cell lung cancer, pancreatic, prostate, bladder, head and neck cancers, sarcomas and ovarian and other gynecological tumors. Combination trials with carboplatin, gemcitabine, pemetrexed, cisplatin, and erlotinib are currently ongoing. Eribulin potentially has a low incidence of peripheral neuropathy. The predominant side effects are neutropenia and fatigue, which are manageable. This article reviews the available information on eribulin with respect to its clinical pharmacology, mechanism of action, pharmacokinetics, pharmacodynamics, metabolism, preclinical studies and clinical trials.
© 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
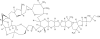

References
-
- Uemura D, Takahashi K, Yamamoto T, et al. Norhalichondrin A: an antitumor polyether macrolide from a marine sponge. J Am Chem Soc. 1985;107(16):4796–8.
-
- Hirata Y, Uemura D. Halichondrins—antitumor polyether macrolides from a marine sponge. Pure Appl Chem. 1986;58(5):701–10.
-
- Pettit GR, Herald CL, Boyd MR, et al. Isolation and structure of the cell growth inhibitory constituents from the western Pacific marine sponge Axinella sp. J Med Chem. 1991;34(11):3339–40. - PubMed
-
- Pettit GR, Tan R, Gao F, et al. Isolation and structure of halistatin 1 from the eastern Indian Ocean marine sponge Phakellia carteri. J Org Chem. 1993;58(9):2538–43.
-
- Gravelos DG, Lake R, Blunt JW, Munro MHG, Litaudon MSP. Halichondrins: cytotoxic polyether macrolides. European Patent Office; Munich, Switzerland: 1993. Publication number EP 0 572 109 A1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

