Histone methyltransferase KMT1A restrains entry of alveolar rhabdomyosarcoma cells into a myogenic differentiated state
- PMID: 21493592
- PMCID: PMC3107367
- DOI: 10.1158/0008-5472.CAN-10-3358
Histone methyltransferase KMT1A restrains entry of alveolar rhabdomyosarcoma cells into a myogenic differentiated state
Abstract
Alveolar rhabdomyosarcoma (ARMS) is an aggressive pediatric muscle cancer, which arrested during the process of skeletal muscle differentiation. In muscle myoblast cells, ectopic expression of the histone H3 lysine 9 (H3K9) methytransferase KMT1A blocks differentiation by repressing a myogenic gene expression program. In this study, we tested the hypothesis that activation of a KMT1A-mediated program of transcriptional repression prevents ARMS cells from differentiating. We investigated whether KMT1A represses the expression of differentiation-associated genes in ARMS cells, thereby blocking muscle differentiation. Our results show that expression of KMT1A is induced in human ARMS cancer cell lines when cultured under differentiation-permissible conditions. shRNA-mediated knockdown of KMT1A decreased anchorage dependent and independent cell proliferation and tumor xenograft growth, increased expression of differentiation-associated genes, and promoted the appearance of a terminally differentiated-like phenotype. Finally, shRNA-directed KMT1A knockdown restored the impaired transcriptional activity of the myogenic regulator MyoD. Together, our results suggested that high levels of KMT1A in ARMS cells under differentiation conditions impairs MyoD function, thereby arresting myogenic differentiation in these tumor cells. Thus, targeting KMT1A may be a novel strategy for the treatment of this disease.
Conflict of interest statement
No potential conflicts of interests were disclosed.
Figures
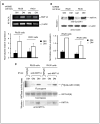
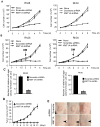
 - represents site of injection).
- represents site of injection).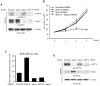
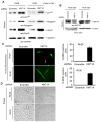

Similar articles
-
p38α MAPK disables KMT1A-mediated repression of myogenic differentiation program.Skelet Muscle. 2016 Aug 22;6:28. doi: 10.1186/s13395-016-0100-z. eCollection 2016. Skelet Muscle. 2016. PMID: 27551368 Free PMC article.
-
Camptothecin exhibits topoisomerase1-independent KMT1A suppression and myogenic differentiation in alveolar rhabdomyosarcoma cells.Oncotarget. 2018 May 25;9(40):25796-25807. doi: 10.18632/oncotarget.25376. eCollection 2018 May 25. Oncotarget. 2018. PMID: 29899822 Free PMC article.
-
SNAIL is a key regulator of alveolar rhabdomyosarcoma tumor growth and differentiation through repression of MYF5 and MYOD function.Cell Death Dis. 2018 May 29;9(6):643. doi: 10.1038/s41419-018-0693-8. Cell Death Dis. 2018. PMID: 29844345 Free PMC article.
-
Alveolar rhabdomyosarcoma-associated proteins PAX3/FOXO1A and PAX7/FOXO1A suppress the transcriptional activity of MyoD-target genes in muscle stem cells.Oncogene. 2013 Jan 31;32(5):651-62. doi: 10.1038/onc.2012.73. Epub 2012 Jun 18. Oncogene. 2013. PMID: 22710712
-
Regulating a master regulator: establishing tissue-specific gene expression in skeletal muscle.Epigenetics. 2010 Nov-Dec;5(8):691-5. doi: 10.4161/epi.5.8.13045. Epub 2010 Nov 1. Epigenetics. 2010. PMID: 20716948 Free PMC article. Review.
Cited by
-
p38α MAPK disables KMT1A-mediated repression of myogenic differentiation program.Skelet Muscle. 2016 Aug 22;6:28. doi: 10.1186/s13395-016-0100-z. eCollection 2016. Skelet Muscle. 2016. PMID: 27551368 Free PMC article.
-
Chaetocin Abrogates the Self-Renewal of Bladder Cancer Stem Cells via the Suppression of the KMT1A-GATA3-STAT3 Circuit.Front Cell Dev Biol. 2020 Jun 17;8:424. doi: 10.3389/fcell.2020.00424. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32626701 Free PMC article.
-
Rhabdomyosarcoma.Nat Rev Dis Primers. 2019 Jan 7;5(1):1. doi: 10.1038/s41572-018-0051-2. Nat Rev Dis Primers. 2019. PMID: 30617281 Free PMC article. Review.
-
Genomic and Epigenetic Changes Drive Aberrant Skeletal Muscle Differentiation in Rhabdomyosarcoma.Cancers (Basel). 2023 May 18;15(10):2823. doi: 10.3390/cancers15102823. Cancers (Basel). 2023. PMID: 37345159 Free PMC article. Review.
-
A method to culture human alveolar rhabdomyosarcoma cell lines as rhabdospheres demonstrates an enrichment in stemness and Notch signaling.Biol Open. 2021 Feb 9;10(2):bio050211. doi: 10.1242/bio.050211. Biol Open. 2021. PMID: 33372065 Free PMC article.
References
-
- Paulino AC, Okcu MF. Rhabdomyosarcoma. Curr Probl Cancer. 2008;32:7–34. - PubMed
-
- Merlino G, Helman LJ. Rhabdomyosarcoma--working out the pathways. Oncogene. 1999;18:5340–8. - PubMed
-
- Tonin PN, Scrable H, Shimada H, Cavenee WK. Muscle-specific gene expression in rhabdomyosarcomas and stages of human fetal skeletal muscle development. Cancer Res. 1991;51:5100–6. - PubMed
-
- Anderson J, Gordon A, Pritchard-Jones K, Shipley J. Genes, chromosomes, and rhabdomyosarcoma. Genes Chromosomes Cancer. 1999;26:275–85. - PubMed
-
- Mercado GE, Xia SJ, Zhang C, Ahn EH, Gustafson DM, Lae M, et al. Identification of PAX3-FKHR-regulated genes differentially expressed between alveolar and embryonal rhabdomyosarcoma: focus on MYCN as a biologically relevant target. Genes Chromosomes Cancer. 2008;47:510–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

