Effect of oxandrolone and timing of pubertal induction on final height in Turner's syndrome: randomised, double blind, placebo controlled trial
- PMID: 21493672
- PMCID: PMC3076731
- DOI: 10.1136/bmj.d1980
Effect of oxandrolone and timing of pubertal induction on final height in Turner's syndrome: randomised, double blind, placebo controlled trial
Abstract
Objective: To examine the effect of oxandrolone and the timing of pubertal induction on final height in girls with Turner's syndrome receiving a standard dose of growth hormone.
Design: Randomised, double blind, placebo controlled trial. Setting 36 paediatric endocrinology departments in UK hospitals.
Participants: Girls with Turner's syndrome aged 7-13 years at recruitment, receiving recombinant growth hormone therapy (10 mg/m(2)/week).
Interventions: Participants were randomised to oxandrolone (0.05 mg/kg/day, maximum 2.5 mg/day) or placebo from 9 years of age. Those with evidence of ovarian failure at 12 years were further randomised to oral ethinylestradiol (year 1, 2 µg daily; year 2, 4 μg daily; year 3, 4 months each of 6, 8, and 10 μg daily) or placebo; participants who received placebo and those recruited after the age of 12.25 years started ethinylestradiol at age 14.
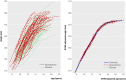
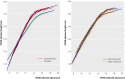
Main outcome measure: Final height. Results 106 participants were recruited, of whom 14 withdrew and 82/92 reached final height. Both oxandrolone and late pubertal induction increased final height: by 4.6 (95% confidence interval 1.9 to 7.2) cm (P = 0.001, n = 82) for oxandrolone and 3.8 (0.0 to 7.5) cm (P = 0.05, n = 48) for late pubertal induction with ethinylestradiol. In the 48 children who were randomised twice, the effects on final height (compared with placebo and early induction of puberty) of oxandrolone alone, late induction alone, and oxandrolone plus late induction were similar, averaging 7.1 (3.4 to 10.8) cm (P < 0.001). No cases of virilisation were reported.
Conclusion: Oxandrolone had a positive effect on final height in girls with Turner's syndrome treated with growth hormone, as did late pubertal induction with ethinylestradiol at age 14 years. However, these effects were not additive, so using both had no advantage. Oxandrolone could, therefore, be offered as an alternative to late pubertal induction for increasing final height in Turner's syndrome. Trial registration Current Controlled Trials ISRCTN50343149.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures

Comment in
-
Puberty induction in Turner syndrome.Nat Rev Endocrinol. 2011 May 24;7(7):375. doi: 10.1038/nrendo.2011.85. Nat Rev Endocrinol. 2011. PMID: 21610688 No abstract available.
References
-
- Karlberg J. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatr 1989;78:70-94. - PubMed
-
- Rochiccioli P, David M, Malpuech G, Colle M, Limal JM, Battin J, et al. Study of final height in Turner’s syndrome: ethnic and genetic influences. Acta Paediatr 1994;83:305-8. - PubMed
-
- Plotnick L, Attie KM, Blethen SL, Sy JP. Growth hormone treatment of girls with Turner syndrome: the National Cooperative Growth Study experience. Pediatrics 1998;102:479-81. - PubMed
-
- Takano K, Shizume K, Hibi I, Ogawa M, Okada Y, Suwa S, et al. Long-term effects of growth hormone treatment on height in Turner syndrome: results of a 6-year multicentre study in Japan. Horm Res 1995;43:141-3. - PubMed
-
- Nilsson KO, Albertsson-Wikland K, Alm J, Aronson S, Gustafsson J, Hagenäs L, et al. Improved final height in girls with Turner’s syndrome treated with growth hormone and oxandrolone. J Clin Endocrinol Metab 1996;81:635-40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
