Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke
- PMID: 21493695
- PMCID: PMC3102237
- DOI: 10.1093/brain/awr063
Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke
Abstract
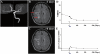
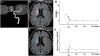
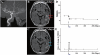
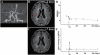
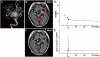
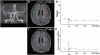
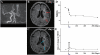
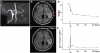
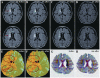
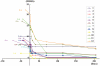
Transplantation of human mesenchymal stem cells has been shown to reduce infarct size and improve functional outcome in animal models of stroke. Here, we report a study designed to assess feasibility and safety of transplantation of autologous human mesenchymal stem cells expanded in autologous human serum in stroke patients. We report an unblinded study on 12 patients with ischaemic grey matter, white matter and mixed lesions, in contrast to a prior study on autologous mesenchymal stem cells expanded in foetal calf serum that focused on grey matter lesions. Cells cultured in human serum expanded more rapidly than in foetal calf serum, reducing cell preparation time and risk of transmissible disorders such as bovine spongiform encephalomyelitis. Autologous mesenchymal stem cells were delivered intravenously 36-133 days post-stroke. All patients had magnetic resonance angiography to identify vascular lesions, and magnetic resonance imaging prior to cell infusion and at intervals up to 1 year after. Magnetic resonance perfusion-imaging and 3D-tractography were carried out in some patients. Neurological status was scored using the National Institutes of Health Stroke Scale and modified Rankin scores. We did not observe any central nervous system tumours, abnormal cell growths or neurological deterioration, and there was no evidence for venous thromboembolism, systemic malignancy or systemic infection in any of the patients following stem cell infusion. The median daily rate of National Institutes of Health Stroke Scale change was 0.36 during the first week post-infusion, compared with a median daily rate of change of 0.04 from the first day of testing to immediately before infusion. Daily rates of change in National Institutes of Health Stroke Scale scores during longer post-infusion intervals that more closely matched the interval between initial scoring and cell infusion also showed an increase following cell infusion. Mean lesion volume as assessed by magnetic resonance imaging was reduced by >20% at 1 week post-cell infusion. While we would emphasize that the current study was unblinded, did not assess overall function or relative functional importance of different types of deficits, and does not exclude placebo effects or a contribution of recovery as a result of the natural history of stroke, our observations provide evidence supporting the feasibility and safety of delivery of a relatively large dose of autologous mesenchymal human stem cells, cultured in autologous human serum, into human subjects with stroke and support the need for additional blinded, placebo-controlled studies on autologous mesenchymal human stem cell infusion in stroke.
Figures






References
-
- Abe K, Hayashi T, Itoyama Y. Amelioration of brain edema by topical application of glial cell line-derived neurotrophic factor in reperfused rat brain. Neurosci Lett. 1997;231:37–40. - PubMed
-
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. - PubMed
-
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–7. - PubMed
-
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. - PubMed
-
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

