Autophagy protects the proximal tubule from degeneration and acute ischemic injury
- PMID: 21493778
- PMCID: PMC3083312
- DOI: 10.1681/ASN.2010070705
Autophagy protects the proximal tubule from degeneration and acute ischemic injury
Abstract
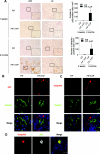
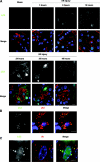
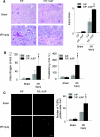
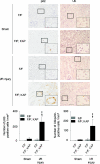
Autophagy is a bulk protein degradation system that likely plays an important role in normal proximal tubule function and recovery from acute ischemic kidney injury. Using conditional Atg5 gene deletion to eliminate autophagy in the proximal tubule, we determined whether autophagy prevents accumulation of damaged proteins and organelles with aging and ischemic renal injury. Autophagy-deficient cells accumulated deformed mitochondria and cytoplasmic inclusions, leading to cellular hypertrophy and eventual degeneration not observed in wildtype controls. In autophagy-deficient mice, I/R injury increased proximal tubule cell apoptosis with accumulation of p62 and ubiquitin positive cytoplasmic inclusions. Compared with control animals, autophagy-deficient mice exhibited significantly greater elevations in serum urea nitrogen and creatinine. These data suggest that autophagy maintains proximal tubule cell homeostasis and protects against ischemic injury. Enhancing autophagy may provide a novel therapeutic approach to minimize acute kidney injury and slow CKD progression.
Copyright © 2011 by the American Society of Nephrology
Figures


References
-
- Mizushima N, Ohsumi Y, Yoshimori T: Autophagosome formation in mammalian cells. Cell Struct Funct 27: 421–429, 2002 - PubMed
-
- Levine B, Klionsky DJ: Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477, 2004 - PubMed
-
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N: Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

