Histone acetylation regulates the cell-specific and interferon-γ-inducible expression of extracellular superoxide dismutase in human pulmonary arteries
- PMID: 21493784
- PMCID: PMC3262691
- DOI: 10.1165/rcmb.2011-0012OC
Histone acetylation regulates the cell-specific and interferon-γ-inducible expression of extracellular superoxide dismutase in human pulmonary arteries
Abstract
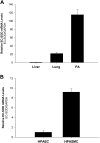
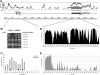
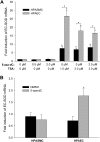
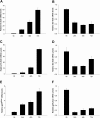
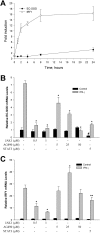
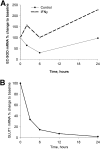
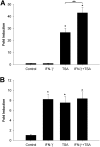
Extracellular superoxide dismutase (EC-SOD) is the major antioxidant enzyme present in the vascular wall, and is responsible for both the protection of vessels from oxidative stress and for the modulation of vascular tone. Concentrations of EC-SOD in human pulmonary arteries are very high relative to other tissues, and the expression of EC-SOD appears highly restricted to smooth muscle. The molecular basis for this smooth muscle-specific expression of EC-SOD is not known. Here we assessed the role of epigenetic factors in regulating the cell-specific and IFN-γ-inducible expression of EC-SOD in human pulmonary artery cells. The analysis of CpG site methylation within the promoter and coding regions of the EC-SOD gene demonstrated higher levels of DNA methylation within the distal promoter region in endothelial cells compared with smooth muscle cells. Exposure of both cell types to DNA demethylation agents reactivated the transcription of EC-SOD in endothelial cells alone. However, exposure to the histone deacetylase inhibitor trichostatin A (TSA) significantly induced EC-SOD gene expression in both endothelial cells and smooth muscle cells. Concentrations of EC-SOD mRNA were also induced up to 45-fold by IFN-γ in smooth muscle cells, but not in endothelial cells. The IFN-γ-dependent expression of EC-SOD was regulated by the Janus tyrosine kinase/signal transducers and activators of transcription proteins signaling pathway. Simultaneous exposure to TSA and IFN-γ produced a synergistic effect on the induction of EC-SOD gene expression, but only in endothelial cells. These findings provide strong evidence that EC-SOD cell-specific and IFN-γ-inducible expression in pulmonary artery cells is regulated, to a major degree, by epigenetic mechanisms that include histone acetylation and DNA methylation.
Figures
Similar articles
-
Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2016 Jul 1;311(1):L124-34. doi: 10.1152/ajplung.00263.2015. Epub 2016 May 27. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27233998 Free PMC article.
-
Regulation of Oxidative Stress in Pulmonary Artery Endothelium. Modulation of Extracellular Superoxide Dismutase and NOX4 Expression Using Histone Deacetylase Class I Inhibitors.Am J Respir Cell Mol Biol. 2015 Oct;53(4):513-24. doi: 10.1165/rcmb.2014-0260OC. Am J Respir Cell Mol Biol. 2015. PMID: 25749103 Free PMC article.
-
Epigenetic regulation of EC-SOD expression in aging lung fibroblasts: Role of histone acetylation.Free Radic Biol Med. 2017 Nov;112:212-223. doi: 10.1016/j.freeradbiomed.2017.07.028. Epub 2017 Jul 27. Free Radic Biol Med. 2017. PMID: 28757400
-
Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins.Arterioscler Thromb Vasc Biol. 1998 Feb;18(2):157-67. doi: 10.1161/01.atv.18.2.157. Arterioscler Thromb Vasc Biol. 1998. PMID: 9484979 Review.
-
Epigenetic regulation of airway inflammation.Curr Opin Immunol. 2007 Dec;19(6):694-700. doi: 10.1016/j.coi.2007.07.016. Epub 2007 Aug 27. Curr Opin Immunol. 2007. PMID: 17720468 Review.
Cited by
-
The Role of Histone Protein Acetylation in Regulating Endothelial Function.Front Cell Dev Biol. 2021 Apr 29;9:672447. doi: 10.3389/fcell.2021.672447. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33996829 Free PMC article. Review.
-
Histone acetylation and arachidonic acid cytotoxicity in HepG2 cells overexpressing CYP2E1.Naunyn Schmiedebergs Arch Pharmacol. 2014 Mar;387(3):271-80. doi: 10.1007/s00210-013-0942-4. Epub 2013 Nov 28. Naunyn Schmiedebergs Arch Pharmacol. 2014. PMID: 24287576 Free PMC article.
-
Akt2 (Protein Kinase B Beta) Stabilizes ATP7A, a Copper Transporter for Extracellular Superoxide Dismutase, in Vascular Smooth Muscle: Novel Mechanism to Limit Endothelial Dysfunction in Type 2 Diabetes Mellitus.Arterioscler Thromb Vasc Biol. 2018 Mar;38(3):529-541. doi: 10.1161/ATVBAHA.117.309819. Epub 2018 Jan 4. Arterioscler Thromb Vasc Biol. 2018. PMID: 29301787 Free PMC article.
-
The dynamic uptake and release of SOD3 from intracellular stores in macrophages modulates the inflammatory response.Redox Biol. 2019 Sep;26:101268. doi: 10.1016/j.redox.2019.101268. Epub 2019 Jul 2. Redox Biol. 2019. PMID: 31326693 Free PMC article.
-
Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension.Am J Physiol Lung Cell Mol Physiol. 2016 Jul 1;311(1):L124-34. doi: 10.1152/ajplung.00263.2015. Epub 2016 May 27. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27233998 Free PMC article.
References
-
- Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol 1995;15:2032–2036 - PubMed
-
- Karlsson K, Marklund SL. Binding of human extracellular-superoxide dismutase C to cultured cell lines and to blood cells. Lab Invest 1989;60:659–666 - PubMed
-
- Abrahamsson T, Brandt U, Marklund SL, Sjoqvist PO. Vascular bound recombinant extracellular superoxide dismutase Type C protects against the detrimental effects of superoxide radicals on endothelium-dependent arterial relaxation. Circ Res 1992;70:264–271 - PubMed
-
- Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II–induced but not catecholamine-induced hypertension. Circulation 1997;95:588–593 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

