Dimethylarginine dimethylaminohydrolase-1 is the critical enzyme for degrading the cardiovascular risk factor asymmetrical dimethylarginine
- PMID: 21493890
- PMCID: PMC3117037
- DOI: 10.1161/ATVBAHA.110.222638
Dimethylarginine dimethylaminohydrolase-1 is the critical enzyme for degrading the cardiovascular risk factor asymmetrical dimethylarginine
Abstract
Objective: The objective of this study was to identify the role of dimethylarginine dimethylaminohydrolase-1 (DDAH1) in degrading the endogenous nitric oxide synthase inhibitors asymmetrical dimethylarginine (ADMA) and N(g)-monomethyl-L-arginine (L-NMMA).
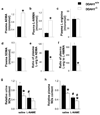
Methods and results: We generated a global-DDAH1 gene-deficient (DDAH1(-/-)) mouse strain to examine the role of DDAH1 in ADMA and l-NMMA degradation and the physiological consequences of loss of DDAH1. Plasma and tissue ADMA and L-NMMA levels in DDAH1(-/-) mice were several folds higher than in wild-type mice, but growth and development of these DDAH1(-/-) mice were similar to those of their wild-type littermates. Although the expression of DDAH2 was unaffected, DDAH activity was undetectable in all tissues tested. These findings indicate that DDAH1 is the critical enzyme for ADMA and L-NMMA degradation. Blood pressure was ≈ 20 mm Hg higher in the DDAH1(-/-) mice than in wild-type mice, but no other cardiovascular phenotype was found under unstressed conditions. Crossing DDAH1(+/-) male with DDAH1(+/-) female mice yielded DDAH1(+/+), DDAH1(+/-), and DDAH1(-/-) mice at the anticipated ratio of 1:2:1, indicating that DDAH1 is not required for embryonic development in this strain.
Conclusions: Our findings indicate that DDAH1 is required for metabolizing ADMA and L-NMMA in vivo, whereas DDAH2 had no detectable role for degrading ADMA and l-NMMA.
Figures
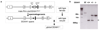
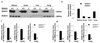


Comment in
-
DDAH says NO to ADMA.Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1462-4. doi: 10.1161/ATVBAHA.111.228833. Arterioscler Thromb Vasc Biol. 2011. PMID: 21677286 Free PMC article. No abstract available.
Similar articles
-
Dimethylarginine dimethylaminohydrolase 1 modulates endothelial cell growth through nitric oxide and Akt.Arterioscler Thromb Vasc Biol. 2011 Apr;31(4):890-7. doi: 10.1161/ATVBAHA.110.215640. Epub 2011 Jan 6. Arterioscler Thromb Vasc Biol. 2011. PMID: 21212404 Free PMC article.
-
DDAH says NO to ADMA.Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1462-4. doi: 10.1161/ATVBAHA.111.228833. Arterioscler Thromb Vasc Biol. 2011. PMID: 21677286 Free PMC article. No abstract available.
-
Dimethylarginine dimethylaminohydrolase-2 deficiency promotes vascular regeneration and attenuates pathological angiogenesis.Exp Eye Res. 2016 Jun;147:148-155. doi: 10.1016/j.exer.2016.05.007. Epub 2016 May 12. Exp Eye Res. 2016. PMID: 27181226 Free PMC article.
-
Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease.Nitric Oxide. 2018 Aug 1;78:113-120. doi: 10.1016/j.niox.2018.06.004. Epub 2018 Jun 19. Nitric Oxide. 2018. PMID: 29928990 Free PMC article. Review.
-
Effect of asymmetric dimethylarginine (ADMA) on heart failure development.Nitric Oxide. 2016 Apr 1;54:73-81. doi: 10.1016/j.niox.2016.02.006. Epub 2016 Feb 24. Nitric Oxide. 2016. PMID: 26923818 Free PMC article. Review.
Cited by
-
The Increasing Problem of Resistant Hypertension: We'll Manage till Help Comes!Med Sci (Basel). 2024 Oct 4;12(4):53. doi: 10.3390/medsci12040053. Med Sci (Basel). 2024. PMID: 39449409 Free PMC article. Review.
-
Biomarcadores de função endotelial em doenças cardiovasculares: hipertensão.J Vasc Bras. 2016 Jul-Sep;15(3):224-233. doi: 10.1590/1677-5449.000316. J Vasc Bras. 2016. PMID: 29930594 Free PMC article. Review. Portuguese.
-
Modulating DDAH/NOS Pathway to Discover Vasoprotective Insulin Sensitizers.J Diabetes Res. 2016;2016:1982096. doi: 10.1155/2016/1982096. Epub 2015 Dec 6. J Diabetes Res. 2016. PMID: 26770984 Free PMC article. Review.
-
Role of ADMA in the pathogenesis of microvascular complications in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2023 Apr 21;14:1183586. doi: 10.3389/fendo.2023.1183586. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37152974 Free PMC article. Review.
-
Packed red blood cells are an abundant and proximate potential source of nitric oxide synthase inhibition.PLoS One. 2015 Mar 20;10(3):e0119991. doi: 10.1371/journal.pone.0119991. eCollection 2015. PLoS One. 2015. PMID: 25793525 Free PMC article.
References
-
- Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Letters. 1994;356:295–298. - PubMed
-
- Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide: Implications for neurodegenerative diseases. FEBS Letters. 1994;345:50–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

