A method to measure myocardial calcium handling in adult Drosophila
- PMID: 21493892
- PMCID: PMC3128985
- DOI: 10.1161/CIRCRESAHA.110.238105
A method to measure myocardial calcium handling in adult Drosophila
Abstract
Rationale: Normal cardiac physiology requires highly regulated cytosolic Ca(2+) concentrations and abnormalities in Ca(2+) handling are associated with heart failure. The majority of approaches to identifying the components that regulate intracellular Ca(2+) dynamics rely on cells in culture, mouse models, and human samples. However, a genetically robust system for unbiased screens of mutations that affect Ca(2+) handling remains a challenge.
Objective: We sought to develop a new method to measure myocardial Ca(2+) cycling in adult Drosophila and determine whether cardiomyopathic fly hearts recapitulate aspects of diseased mammalian myocardium.
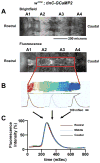
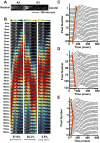
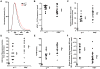
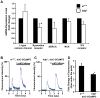
Methods and results: Using engineered transgenic Drosophila that have cardiac-specific expression of Ca(2+)-sensing fluorescent protein, GCaMP2, we developed methods to measure parameters associated with myocardial Ca(2+) handling. The following key observations were identified: (1) Control w(1118) Drosophila hearts have readily measureable Ca(2+)-dependent fluorescent signals that are dependent on L-type Ca(2+) channels and SR Ca(2+) stores and originate from rostral and caudal pacemakers. (2) A fly mutant, held-up(2) (hdp(2)), that has a point mutation in troponin I and has a dilated cardiomyopathic phenotype demonstrates abnormalities in myocardial Ca(2+) handling that include increases in the duration of the 50% rise in intensity to peak intensity, the half-time of fluorescence decline from peak, the full duration at half-maximal intensity, and decreases in the linear slope of decay from 80% to 20% intensity decay. (3) Hearts from hdp(2) mutants had reductions in caffeine-induced Ca(2+) increases and reductions in ryanodine receptor (RyR) without changes in L-type Ca(2+) channel transcripts in comparison with w(1118).
Conclusions: Our results show that the cardiac-specific expression of GCaMP2 provides a means of characterizing propagating Ca(2+) transients in adult fly hearts. Moreover, the adult fruit fly heart recapitulates several aspects of Ca(2+) regulation observed in mammalian myocardium. A mutation in Drosophila that causes an enlarged cardiac chamber and impaired contractile function is associated with abnormalities in the cytosolic Ca(2+) transient as well as changes in transcript levels of proteins associated with Ca(2+) handling. This new methodology has the potential to permit an examination of evolutionarily conserved myocardial Ca(2+)-handing mechanisms by applying the vast resources available in the fly genomics community to conduct genetic screens to identify new genes involved in generated Ca(2+) transients and arrhythmias.
Figures


Similar articles
-
Conservation of cardiac L-type Ca2+ channels and their regulation in Drosophila: A novel genetically-pliable channelopathic model.J Mol Cell Cardiol. 2018 Jun;119:64-74. doi: 10.1016/j.yjmcc.2018.04.010. Epub 2018 Apr 21. J Mol Cell Cardiol. 2018. PMID: 29684406 Free PMC article.
-
Calcium-sensitivity of the SR calcium release channel in failing and nonfailing human myocardium.Basic Res Cardiol. 1999 Jun;94(3):145-51. doi: 10.1007/s003950050137. Basic Res Cardiol. 1999. PMID: 10424232
-
Ca(2+) signaling in cardiac myocytes overexpressing the alpha(1) subunit of L-type Ca(2+) channel.Circ Res. 2002 Feb 8;90(2):174-81. doi: 10.1161/hh0202.103230. Circ Res. 2002. PMID: 11834710
-
Mouse Models of Cardiomyopathies Caused by Mutations in Troponin C.Int J Mol Sci. 2023 Aug 2;24(15):12349. doi: 10.3390/ijms241512349. Int J Mol Sci. 2023. PMID: 37569724 Free PMC article. Review.
-
Integrative analysis of calcium signalling in cardiac muscle.Front Biosci. 2002 Apr 1;7:d843-52. doi: 10.2741/trafford. Front Biosci. 2002. PMID: 11897570 Review.
Cited by
-
Modeling dilated cardiomyopathies in Drosophila.Trends Cardiovasc Med. 2012 Apr;22(3):55-61. doi: 10.1016/j.tcm.2012.06.012. Epub 2012 Aug 3. Trends Cardiovasc Med. 2012. PMID: 22863366 Free PMC article.
-
A Drosophila melanogaster model of diastolic dysfunction and cardiomyopathy based on impaired troponin-T function.Circ Res. 2014 Jan 17;114(2):e6-17. doi: 10.1161/CIRCRESAHA.114.302028. Epub 2013 Nov 12. Circ Res. 2014. PMID: 24221941 Free PMC article.
-
SERCA is critical to control the Bowditch effect in the heart.Sci Rep. 2018 Aug 20;8(1):12447. doi: 10.1038/s41598-018-30638-9. Sci Rep. 2018. PMID: 30127403 Free PMC article.
-
Interplay between SERCA, 4E-BP, and eIF4E in the Drosophila heart.PLoS One. 2022 May 19;17(5):e0267156. doi: 10.1371/journal.pone.0267156. eCollection 2022. PLoS One. 2022. PMID: 35588119 Free PMC article.
-
Neprilysins regulate muscle contraction and heart function via cleavage of SERCA-inhibitory micropeptides.Nat Commun. 2022 Jul 29;13(1):4420. doi: 10.1038/s41467-022-31974-1. Nat Commun. 2022. PMID: 35906206 Free PMC article.
References
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
-
- Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci. 2005;1047:86–98. - PubMed
-
- Bers DM. Altered cardiac myocyte ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. - PubMed
-
- Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. - PubMed
-
- Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

