Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy
- PMID: 21493893
- PMCID: PMC3109741
- DOI: 10.1161/CIRCRESAHA.110.239848
Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy
Abstract
Rationale: Bone marrow (BM)-derived mesenchymal stem cells (MSCs) hold great promise for cardiovascular cell therapy owing to their multipotency and culture expandability.
Objective: The aim of the study was to investigate whether MSCs can treat experimental acute myocardial infarction (MI) and diabetic neuropathy.
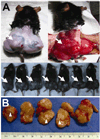
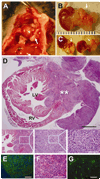
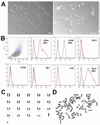
Methods and results: We isolated mononuclear cells from mouse BM and cultured MSCs in a conventional manner. Flow cytometry analyses of these cultured cells at passage 4 showed expression of typical MSC markers such as CD44 and CD29, but not hematopoietic markers such as c-kit, flk1, and CD34. To determine the therapeutic effects of MSCs, we injected MSCs into the peri-infarct area after ligation of the left anterior descending coronary arteries of mice and, as separate experiments, injected the same batch of MSCs into hindlimb muscles of mice with diabetic neuropathy. During the follow-up at 4 to 8 weeks after cell transplantation, growing tumors were observed in 30% of hearts in the MI model, and in 46% of hindlimbs in the diabetic neuropathy model. Histological examination of the tumors revealed hypercelluarity, pleomorphic nucleoli, cytological atypia and necrosis, and positive staining for α-smooth muscle actin, indicative of malignant sarcoma with myogenic differentiation. Chromosomal analysis of these MSCs showed multiple chromosomal aberrations including fusion, fragmentation, and ring formation.
Conclusions: Genetically unmodified MSCs can undergo chromosomal abnormalities even at early passages and form malignant tumors when transplanted in vivo. These results suggest that careful monitoring of chromosomal status is warranted when in vitro expanded MSCs are used for cell therapy such as for MI.
Figures

Comment in
-
What is the oncologic risk of stem cell treatment for heart disease?Circ Res. 2011 May 27;108(11):1300-3. doi: 10.1161/CIRCRESAHA.111.246611. Circ Res. 2011. PMID: 21617132 Free PMC article. No abstract available.
Similar articles
-
C-Kit Positive Cardiac Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner.J Card Fail. 2017 May;23(5):403-415. doi: 10.1016/j.cardfail.2017.03.002. Epub 2017 Mar 8. J Card Fail. 2017. PMID: 28284757
-
Effects of hepatocyte growth factor overexpressed bone marrow-derived mesenchymal stem cells on prevention from left ventricular remodelling and functional improvement in infarcted rat hearts.Cell Biochem Funct. 2012 Oct;30(7):574-81. doi: 10.1002/cbf.2836. Epub 2012 May 16. Cell Biochem Funct. 2012. PMID: 22592978
-
Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells.Stem Cells. 2007 Mar;25(3):612-20. doi: 10.1634/stemcells.2006-0168. Epub 2006 Nov 9. Stem Cells. 2007. PMID: 17095707
-
Experimental study of bone marrow-derived mesenchymal stem cells combined with hepatocyte growth factor transplantation via noninfarct-relative artery in acute myocardial infarction.Gene Ther. 2006 Nov;13(22):1564-8. doi: 10.1038/sj.gt.3302820. Epub 2006 Jun 29. Gene Ther. 2006. PMID: 16810195
-
Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction.Circ Res. 2011 Feb 18;108(4):478-89. doi: 10.1161/CIRCRESAHA.110.229658. Epub 2011 Jan 13. Circ Res. 2011. PMID: 21233455 Free PMC article.
Cited by
-
Monomeric CXCL12-Engineered Adipose-Derived Stem Cells Transplantation for the Treatment of Ischemic Stroke.Int J Mol Sci. 2024 Jan 8;25(2):792. doi: 10.3390/ijms25020792. Int J Mol Sci. 2024. PMID: 38255866 Free PMC article.
-
Cellular therapies for idiopathic pulmonary fibrosis: current progress and future prospects.Am J Stem Cells. 2024 Aug 25;13(4):191-211. doi: 10.62347/DAKS5508. eCollection 2024. Am J Stem Cells. 2024. PMID: 39308764 Free PMC article. Review.
-
Enhancing osteoporosis treatment with engineered mesenchymal stem cell-derived extracellular vesicles: mechanisms and advances.Cell Death Dis. 2024 Feb 8;15(2):119. doi: 10.1038/s41419-024-06508-w. Cell Death Dis. 2024. PMID: 38331884 Free PMC article. Review.
-
Discrepancy between short-term and long-term effects of bone marrow-derived cell therapy in acute myocardial infarction: a systematic review and meta-analysis.Stem Cell Res Ther. 2016 Oct 20;7(1):153. doi: 10.1186/s13287-016-0415-z. Stem Cell Res Ther. 2016. PMID: 27765070 Free PMC article.
-
Cardiac stem cells and their roles in myocardial infarction.Stem Cell Rev Rep. 2013 Jun;9(3):326-38. doi: 10.1007/s12015-012-9421-4. Stem Cell Rev Rep. 2013. PMID: 23238707 Review.
References
-
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. - PubMed
-
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. - PubMed
-
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. - PubMed
-
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

