Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells
- PMID: 21494253
- PMCID: PMC4175714
- DOI: 10.1038/leu.2011.62
Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells
Erratum in
- Leukemia. 2011 Aug;25(8):1378
Abstract
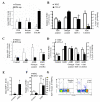
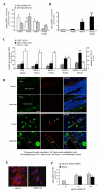
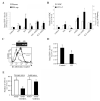
Steady-state egress of hematopoietic progenitor cells can be rapidly amplified by mobilizing agents such as AMD3100, the mechanism, however, is poorly understood. We report that AMD3100 increased the homeostatic release of the chemokine stromal cell derived factor-1 (SDF-1) to the circulation in mice and non-human primates. Neutralizing antibodies against CXCR4 or SDF-1 inhibited both steady state and AMD3100-induced SDF-1 release and reduced egress of murine progenitor cells over mature leukocytes. Intra-bone injection of biotinylated SDF-1 also enhanced release of this chemokine and murine progenitor cell mobilization. AMD3100 directly induced SDF-1 release from CXCR4(+) human bone marrow osteoblasts and endothelial cells and activated uPA in a CXCR4/JNK-dependent manner. Additionally, ROS inhibition reduced AMD3100-induced SDF-1 release, activation of circulating uPA and mobilization of progenitor cells. Norepinephrine treatment, mimicking acute stress, rapidly increased SDF-1 release and progenitor cell mobilization, whereas β2-adrenergic antagonist inhibited both steady state and AMD3100-induced SDF-1 release and progenitor cell mobilization in mice. In conclusion, this study reveals that SDF-1 release from bone marrow stromal cells to the circulation emerges as a pivotal mechanism essential for steady-state egress and rapid mobilization of hematopoietic progenitor cells, but not mature leukocytes.
Figures

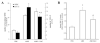
References
-
- Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–92. - PubMed
-
- Lapidot T, Kollet O. Hematology. 30th edition 2010. The Brain-Bone-Blood Triad: Traffic Lights for Stem-Cell Homing and Mobilization. - PubMed
-
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–6. - PubMed
-
- Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. - PubMed
-
- Lapid K, Vagima Y, Kollet O, Lapidot T. Egress and mobilization of hematopoietic progenitor cells. StemBook, Harvard Stem Cell Institute. 2009 1.39.1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

